Chloromethylation Modified Pyranonitrile-Based Conjugated Microporous Polymers for Selective One-Step Two-Electron O2 Reduction to H2O2
Graphical Abstract
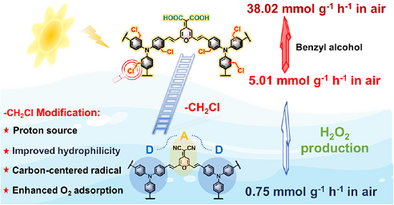
A post-modification strategy employing a chloromethylation reaction was proposed to enhance H2O2 production of covalent microporous polymers (CMPs). The H2O2 production rate of the chloromethylated CMP namely DCM-TPA-Cl reached 5.01 mmol g−1 h−1 in air, which is 6.7 times greater than that of the unmodified photocatalyst. An exceptional 38.02 mmol g−1 h−1 rate was further achieved in water/benzyl alcohol mixtures, exceeding that of most reported polymer photocatalysts.
Abstract
Hydrogen peroxide (H2O2) production utilizing conjugated microporous polymers (CMPs)-based photocatalysts represents a crucial green technology for achieving solar-to-chemical energy conversion. Proper material design is paramount to improve the dispersity and charge transfer of CMPs for enhanced H2O2 production performance. Herein, a post-modification strategy employing chloromethylation reaction was proposed to enhance H2O2 production. The simple one-step chloromethylation reaction simultaneously achieved two objectives: One is enhanced hydrophilicity through the hydrolysis of cyanogen groups in the pyranonitrile unit to carboxyl groups, the other is the improved O2 adsorption and charge transfer by incorporating chloromethyl groups. The two objectives synergistically enhanced the H2O2 production rate of the chloromethylated CMP named DCM-TPA-Cl, reaching 5.01 mmol g−1 h−1 in air, 6.7-fold of the unmodified photocatalyst. Moreover, the rate achieved at an O2 atmosphere increased by only 1.8%, highlighting its superior O2 utilization efficiency in air. An exceptional 38.02 mmol g−1 h−1 rate was further achieved in water/benzyl alcohol mixtures, exceeding most reported polymer photocatalysts. Experimental and theoretical results corroborated the predominant role of the one-step two-electron O2 reduction pathway in the H2O2 generation. This work demonstrates the potential of a post-modification method to significantly enhance H2O2 production performance directly from water and air.
Conflict of Interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the Supporting Information of this article.