Ternary Aldehyde–Copper–Iridium Catalysis Enables Stereodivergent Allylation via α-C-H Functionalization of Primary Amines
Zijiao Liu
Shanghai University of Medicine & Health Sciences Affiliated Sixth People's Hospital South Campus, Shanghai, 201499 China
Shanghai Key Laboratory for Molecular Engineering of Chiral Drugs, State Key Laboratory of Synergistic Chem-Bio Synthesis, Frontiers Science Center for Transformative Molecules, School of Chemistry and Chemical Engineering, Shanghai Jiao Tong University, Shanghai, 200240 China
Both authors contributed equally to this work.
Search for more papers by this authorDr. Panpan Li
Shanghai Key Laboratory for Molecular Engineering of Chiral Drugs, State Key Laboratory of Synergistic Chem-Bio Synthesis, Frontiers Science Center for Transformative Molecules, School of Chemistry and Chemical Engineering, Shanghai Jiao Tong University, Shanghai, 200240 China
Both authors contributed equally to this work.
Search for more papers by this authorHaoyang Wang
Shanghai Key Laboratory for Molecular Engineering of Chiral Drugs, State Key Laboratory of Synergistic Chem-Bio Synthesis, Frontiers Science Center for Transformative Molecules, School of Chemistry and Chemical Engineering, Shanghai Jiao Tong University, Shanghai, 200240 China
Search for more papers by this authorDr. Jiacheng Zhang
Shanghai Key Laboratory for Molecular Engineering of Chiral Drugs, State Key Laboratory of Synergistic Chem-Bio Synthesis, Frontiers Science Center for Transformative Molecules, School of Chemistry and Chemical Engineering, Shanghai Jiao Tong University, Shanghai, 200240 China
Search for more papers by this authorCorresponding Author
Prof. Xiaohong Huo
Shanghai Key Laboratory for Molecular Engineering of Chiral Drugs, State Key Laboratory of Synergistic Chem-Bio Synthesis, Frontiers Science Center for Transformative Molecules, School of Chemistry and Chemical Engineering, Shanghai Jiao Tong University, Shanghai, 200240 China
E-mail: [email protected]; [email protected]; [email protected]
Search for more papers by this authorCorresponding Author
Prof. Zhen-Liang Sun
Shanghai University of Medicine & Health Sciences Affiliated Sixth People's Hospital South Campus, Shanghai, 201499 China
E-mail: [email protected]; [email protected]; [email protected]
Search for more papers by this authorCorresponding Author
Prof. Wanbin Zhang
Shanghai Key Laboratory for Molecular Engineering of Chiral Drugs, State Key Laboratory of Synergistic Chem-Bio Synthesis, Frontiers Science Center for Transformative Molecules, School of Chemistry and Chemical Engineering, Shanghai Jiao Tong University, Shanghai, 200240 China
E-mail: [email protected]; [email protected]; [email protected]
Search for more papers by this authorZijiao Liu
Shanghai University of Medicine & Health Sciences Affiliated Sixth People's Hospital South Campus, Shanghai, 201499 China
Shanghai Key Laboratory for Molecular Engineering of Chiral Drugs, State Key Laboratory of Synergistic Chem-Bio Synthesis, Frontiers Science Center for Transformative Molecules, School of Chemistry and Chemical Engineering, Shanghai Jiao Tong University, Shanghai, 200240 China
Both authors contributed equally to this work.
Search for more papers by this authorDr. Panpan Li
Shanghai Key Laboratory for Molecular Engineering of Chiral Drugs, State Key Laboratory of Synergistic Chem-Bio Synthesis, Frontiers Science Center for Transformative Molecules, School of Chemistry and Chemical Engineering, Shanghai Jiao Tong University, Shanghai, 200240 China
Both authors contributed equally to this work.
Search for more papers by this authorHaoyang Wang
Shanghai Key Laboratory for Molecular Engineering of Chiral Drugs, State Key Laboratory of Synergistic Chem-Bio Synthesis, Frontiers Science Center for Transformative Molecules, School of Chemistry and Chemical Engineering, Shanghai Jiao Tong University, Shanghai, 200240 China
Search for more papers by this authorDr. Jiacheng Zhang
Shanghai Key Laboratory for Molecular Engineering of Chiral Drugs, State Key Laboratory of Synergistic Chem-Bio Synthesis, Frontiers Science Center for Transformative Molecules, School of Chemistry and Chemical Engineering, Shanghai Jiao Tong University, Shanghai, 200240 China
Search for more papers by this authorCorresponding Author
Prof. Xiaohong Huo
Shanghai Key Laboratory for Molecular Engineering of Chiral Drugs, State Key Laboratory of Synergistic Chem-Bio Synthesis, Frontiers Science Center for Transformative Molecules, School of Chemistry and Chemical Engineering, Shanghai Jiao Tong University, Shanghai, 200240 China
E-mail: [email protected]; [email protected]; [email protected]
Search for more papers by this authorCorresponding Author
Prof. Zhen-Liang Sun
Shanghai University of Medicine & Health Sciences Affiliated Sixth People's Hospital South Campus, Shanghai, 201499 China
E-mail: [email protected]; [email protected]; [email protected]
Search for more papers by this authorCorresponding Author
Prof. Wanbin Zhang
Shanghai Key Laboratory for Molecular Engineering of Chiral Drugs, State Key Laboratory of Synergistic Chem-Bio Synthesis, Frontiers Science Center for Transformative Molecules, School of Chemistry and Chemical Engineering, Shanghai Jiao Tong University, Shanghai, 200240 China
E-mail: [email protected]; [email protected]; [email protected]
Search for more papers by this authorGraphical Abstract
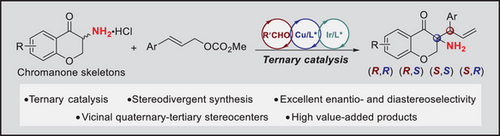
A direct enantio- and diastereodivergent α-allylation of unprotected primary amines has been developed using aldehyde/Cu/Ir ternary catalysis. This catalytic system enables the synthesis of α-tertiary primary amines bearing vicinal stereocenters in high yields with excellent stereoselectivities. Notably, this method establishes a sophisticated asymmetric induction model for asymmetric α-C-H functionalization of unprotected primary amines.
Abstract
α-Chiral primary amines are recognized as one of the most valuable and versatile synthetic intermediates, widely utilized in the construction of diverse amine-containing natural products, pharmaceuticals, and agrochemicals. The direct asymmetric α-C-H functionalization of unprotected primary amines is the most straightforward method for creating these motifs. However, this transformation remains underdeveloped, particularly in stereodivergent synthesis of primary amines with multiple stereocenters. Herein, we report an aldehyde/copper/iridium ternary catalytic system, which was successfully employed for the direct enantio- and diastereodivergent α-allylation of primary α-amino-chromanone without requiring additional protection or activation of the NH2 group. A wide range of α-tertiary primary amines bearing vicinal stereocenters were prepared in high yields with excellent enantio- and diastereoselectivities (generally >20:1 dr and >99% ee). Notably, all four stereoisomers of the α-tertiary amines can be readily prepared by simply switching the configuration combinations of the two chiral metal catalysts. Furthermore, the asymmetric induction model for the α-C-H functionalization of primary amines was meticulously elucidated through comprehensive density functional theory (DFT) calculations.
Conflict of Interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.
Supporting Information
| Filename | Description |
|---|---|
| anie202508335-sup-0001-SuppMat.pdf15.1 MB | Supporting Information |
| anie202508335-sup-0002-SuppMat1.cif544.2 KB | Supporting Information |
| anie202508335-sup-0003-SuppMat2.cif661.5 KB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1T. C. Nugent, Chiral Amine Synthesis: Methods, Developments and Applications, Wiley-VCH, Weinheim, 2010.
10.1002/9783527629541 Google Scholar
- 2E. Vitaku, D. T. Smith, J. T. Njardarson, J. Med. Chem. 2014, 57, 10257–10274.
- 3A. Trowbridge, S. M. Walton, M. J. Gaunt, Chem. Rev. 2020, 120, 2613–2692.
- 4A. Ricci, L. Bernardi, Methodologies in Amine Synthesis: Challenges and Applications, Wiley-VCH, Weinheim, 2021.
10.1002/9783527826186 Google Scholar
- 5Njardarson's group https://njardarson.lab.arizona.edu/content/toppharmaceuticals-poster.
- 6For selected reviews, see: J. Michaux, G. Niel, J.-M. Campagne, Chem. Soc. Rev. 2009, 38, 2093.
- 7S. D. Roughley, A. M. Jordan, J. Med. Chem. 2011, 54, 3451–3479.
- 8Q. Yin, Y. Shi, J. Wang, X. Zhang, Chem. Soc. Rev. 2020, 49, 6141–6153.
- 9B. Waldeck, Chirality 1993, 5, 350–355.
- 10K. M. Rentsch, J. Biochem. Biophys. Methods 2002, 54, 1–9.
- 11K. Jozwiak, W. J. Lough, I. W. Wainer, Drug Stereochemistry: Analytical Methods and Pharmacology, 3rd ed., Informa, New York, 2012.
10.3109/9781420092394 Google Scholar
- 12L. Lin, X. Feng, Chem. - Eur. J. 2017, 23, 6464–6482.
- 13S. Krautwald, E. M. Carreira, J. Am. Chem. Soc. 2017, 139, 5627–5639.
- 14I. P. Beletskaya, C. Nájera, M. Yus, Chem. Rev. 2018, 118, 5080–5200.
- 15X. Huo, G. Li, X. Wang, W. Zhang, Angew. Chem. Int. Ed. 2022, 61, e202210086.
- 16D.-F. Chen, L.-Z. Gong, J. Am. Chem. Soc. 2022, 144, 2415–2437.
- 17L. Wei, C. Fu, Z.-F. Wang, H.-Y. Tao, C.-J. Wang, ACS Catal. 2024, 14, 3812–3844.
- 18H. Wang, Q. Zhang, W. Zi, Acc. Chem. Res. 2024, 57, 468–488.
- 19For the pioneering works of metal/organo catalysis in stereodivergent synthesis, see: S. Krautwald, D. Sarlah, M. A. Schafroth, E. M. Carreira, Science 2013, 340, 1065–1068.
- 20S. Krautwald, M. A. Schafroth, D. Sarlah, E. M. Carreira, J. Am. Chem. Soc. 2014, 136, 3020–3023.
- 21For the pioneering work of organo/organo catalysis in stereodivergent synthesis, see: B. Kim, Y. Kim, S. Y. Lee, J. Am. Chem. Soc. 2021, 143, 73–79.
- 22S.-L. Shi, Z. L. Wong, S. L. Buchwald, Nature 2016, 532, 353–356.
- 23S. Zhang, J. del Pozo, F. Romiti, Y. Mu, S. Torker, A. H. Hoveyda, Science 2019, 364, 45–51.
- 24W. Wen, M.-J. Luo, Y. Yuan, J.-H. Liu, Z.-L. Wu, T. Cai, Z.-W. Wu, Q. Ouyang, Q.-X. Guo, Nat. Commun. 2020, 11, 5372.
- 25Q. Zhou, Z.-W. Yin, Z.-L. Wu, T. Cai, W. Wen, Y.-M. Huang, Q.-X. Guo, Org. Lett. 2023, 25, 5790–5794.
- 26M.-L. Li, J.-H. Yu, Y.-H. Li, S.-F. Zhu, Q.-L. Zhou, Science 2019, 366, 990–994.
- 27S. Z. Ali, B. G. Budaitis, D. F. A. Fontaine, A. L. Pace, J. A. Garwin, M. C. White, Science 2022, 376, 276–283.
- 28K. P. S. Cheung, J. Fang, K. Mukherjee, A. Mihranyan, V. Gevorgyan, Science 2022, 378, 1207–1213.
- 29Y. Jin, Y. Jing, C. Li, M. Li, W. Wu, Z. Ke, H. Jiang, Nat. Chem. 2022, 14, 1118–1125.
- 30J.-J. Chen, J.-H. Fang, X.-Y. Du, J.-Y. Zhang, J.-Q. Bian, F.-L. Wang, C. Luan, W.-L. Liu, J.-R. Liu, X.-Y. Dong, Z.-L. Li, Q.-S. Gu, Z. Dong, X.-Y. Liu, Nature 2023, 618, 294–300.
- 31J. P. Wolfe, S. Wagaw, J.-F. Marcoux, S. L. Buchwald, Acc. Chem. Res. 1998, 31, 805–818.
- 32J. F. Hartwig, Angew. Chem. Int. Ed. 1998, 37, 2046–2067.
10.1002/(SICI)1521-3773(19980817)37:15<2046::AID-ANIE2046>3.0.CO;2-L CAS PubMed Web of Science® Google Scholar
- 33J. F. Hartwig, Acc. Chem. Res. 1998, 31, 852–860.
- 34J. F. Hartwig, Nature 2008, 455, 314–322.
- 35D. S. Surry, S. L. Buchwald, Chem. Sci. 2011, 2, 27–50.
- 36J. Bariwal, E. Van der Eycken, Chem. Soc. Rev. 2013, 42, 9283.
- 37P. Ruiz-Castillo, S. L. Buchwald, Chem. Rev. 2016, 116, 12564–12649.
- 38S. Bhunia, G. G. Pawar, S. V. Kumar, Y. Jiang, D. Ma, Angew. Chem. Int. Ed. 2017, 56, 16136–16179.
- 39X. Q. Ng, C. S. Lim, M. W. Liaw, T. T. Quach, B.-M. Yang, V. Isoni, J. Wu, Y. Zhao, Nat. Synth. 2023, 2, 572–580.
- 40Y. Gao, G. Hong, B. M. Yang, Y. Zhao, Chem. Soc. Rev. 2023, 52, 5541–5562.
- 41J. F. Hartwig, L. M. Stanley, Acc. Chem. Res. 2010, 43, 1461–1475.
- 42J. C. Hethcox, S. E. Shockley, B. M. Stoltz, ACS Catal. 2016, 6, 6207–6213.
- 43J. Qu, G. Helmchen, Acc. Chem. Res. 2017, 50, 2539–2555.
- 44Q. Cheng, H.-F. Tu, C. Zheng, J.-P. Qu, G. Helmchen, S.-L. You, Chem. Rev. 2019, 119, 1855–1969.
- 45S. L. Rössler, D. A. Petrone, E. M. Carreira, Acc. Chem. Res. 2019, 52, 2657–2672.
- 46C. E. Stivala, J. R. Zbieg, P. Liu, M. J. Krische, Acc. Chem. Res. 2022, 55, 2138–2147.
- 47T. Ohmura, J. F. Hartwig, J. Am. Chem. Soc. 2002, 124, 15164–15165.
- 48K. Tissot-Croset, D. Polet, A. Alexakis, Angew. Chem. Int. Ed. 2004, 43, 2426–2428.
- 49C. Welter, A. Dahnz, B. Brunner, S. Streiff, P. Dübon, G. Helmchen, Org. Lett. 2005, 7, 1239–1242.
- 50Y. Yamashita, A. Gopalarathnam, J. F. Hartwig, J. Am. Chem. Soc. 2007, 129, 7508–7509.
- 51K.-Y. Ye, Z.-A. Zhao, Z.-W. Lai, L.-X. Dai, S.-L. You, Synthesis 2013, 45, 2109–2114.
- 52C. C. Malakar, G. Helmchen, Chem. - Eur. J. 2015, 21, 7127–7134.
- 53A. T. Meza, T. Wurm, L. Smith, S. W. Kim, J. R. Zbieg, C. E. Stivala, M. J. Krische, J. Am. Chem. Soc. 2018, 140, 1275–1279.
- 54S. W. Kim, L. A. Schwartz, J. R. Zbieg, C. E. Stivala, M. J. Krische, J. Am. Chem. Soc. 2019, 141, 671–676.
- 55J. Li, S. Gong, S. Gao, J. Chen, W.-W. Chen, B. Zhao, Nat. Commun. 2024, 15, 939.
- 56D. A. Horton, G. T. Bourne, M. L. Smythe, Chem. Rev. 2003, 103, 893–930.
- 57A. Gaspar, M. J. Matos, J. Garrido, E. Uriarte, F. Borges, Chem. Rev. 2014, 114, 4960–4992.
- 58L. Feng, M. M. Maddox, M. Z. Alam, L. S. Tsutsumi, G. Narula, D. F. Bruhn, X. Wu, S. Sandhaus, R. B. Lee, C. J. Simmons, Y.-C. Tse-Dinh, J. G. Hurdle, R. E. Lee, D. Sun, J. Med. Chem. 2014, 57, 8398–8420.
- 59T. Seifert, M. Malo, T. Kokkola, K. Engen, M. Fridén-Saxin, E. A. A. Wallén, M. Lahtela-Kakkonen, E. M. Jarho, K. Luthman, J. Med. Chem. 2014, 57, 9870–9888.
- 60J. Reis, A. Gaspar, N. Milhazes, F. Borges, J. Med. Chem. 2017, 60, 7941–7957.
- 61D. Huckle, I. M. Lockhart, M. Wright, J. Med. Chem. 1969, 12, 277–279.
- 62H. A. DeWald, T. G. Heffner, J. C. Jaen, D. M. Lustgarten, A. T. McPhail, L. T. Meltzer, T. A. Pugsley, L. D. Wise, J. Med. Chem. 1990, 33, 445–450.
- 63L. A. van Vliet, N. Rodenhuis, H. Wikström, T. A. Pugsley, K. A. Serpa, L. T. Meltzer, T. G. Heffner, L. D. Wise, M. E. Lajiness, R. M. Huff, K. Svensson, G. R. M. M. Haenen, A. Bast, J. Med. Chem. 2000, 43, 3549–3557.
- 64N. T. Hatzenbuhler, R. Baudy, D. A. Evrard, A. Failli, B. L. Harrison, S. Lenicek, R. E. Mewshaw, A. Saab, U. Shah, J. Sze, M. Zhang, D. Zhou, M. Chlenov, M. Kagan, J. Golembieski, G. Hornby, M. Lai, D. L. Smith, K. M. Sullivan, L. E. Schechter, T. H. Andree, J. Med. Chem. 2008, 51, 6980–7004.
- 65S. Hudson, M. Kiankarimi, W. Eccles, Y. S. Mostofi, M. J. Genicot, W. Dwight, B. A. Fleck, K. Gogas, W. S. Wade, Bioorg. Med. Chem. Lett. 2008, 18, 4495–4498.
- 66P. A. Harris, S. B. Berger, J. U. Jeong, R. Nagilla, D. Bandyopadhyay, N. Campobasso, C. A. Capriotti, J. A. Cox, L. Dare, X. Dong, P. M. Eidam, J. N. Finger, S. J. Hoffman, J. Kang, V. Kasparcova, B. W. King, R. Lehr, Y. Lan, L. K. Leister, J. D. Lich, T. T. MacDonald, N. A. Miller, M. T. Ouellette, C. S. Pao, A. Rahman, M. A. Reilly, A. R. Rendina, E. J. Rivera, M. C. Schaeffer, C. A. Sehon, R. R. Singhaus, H. H. Sun, B. A. Swift, R. D. Totoritis, A. Vossenkämper, P. Ward, D. D. Wisnoski, D. Zhang, R. W. Marquis, P. J. Gough, J. Bertin, J. Med. Chem. 2017, 60, 1247–1261.
- 67T. Imaeda, K. Ono, K. Nakai, Y. Hori, J. Matsukawa, T. Takagi, Y. Fujioka, N. Tarui, M. Kondo, A. Imanishi, N. Inatomi, M. Kajino, F. Itoh, H. Nishida, Biorg. Med. Chem. 2017, 25, 3719–3735.
- 68X. Huo, R. He, X. Zhang, W. Zhang, J. Am. Chem. Soc. 2016, 138, 11093–11096.
- 69R. He, X. Huo, L. Zhao, F. Wang, L. Jiang, J. Liao, W. Zhang, J. Am. Chem. Soc. 2020, 142, 8097–8103.
- 70J. Zhang, X. Huo, J. Xiao, L. Zhao, S. Ma, W. Zhang, J. Am. Chem. Soc. 2021, 143, 12622–12632.
- 71Y. Peng, C. Han, Y. Luo, G. Li, X. Huo, W. Zhang, Angew. Chem. Int. Ed. 2022, 61, e202203448.
- 72Y. Luo, Y. Ma, G. Li, X. Huo, W. Zhang, Angew. Chem. Int. Ed. 2023, 62, e202313838.
- 73W. Zhu, C. Han, G. Yang, X. Huo, W. Zhang, J. Am. Chem. Soc. 2024, 146, 26121–26130.
- 74P. Li, E. Zheng, G. Li, Y. Luo, X. Huo, S. Ma, W. Zhang, Science 2024, 385, 972–979.
- 75X. Jiang, P. Boehm, J. F. Hartwig, J. Am. Chem. Soc. 2018, 140, 1239–1242.
- 76L. Wei, Q. Zhu, S.-M. Xu, X. Chang, C.-J. Wang, J. Am. Chem. Soc. 2018, 140, 1508–1513.
- 77Z.-T. He, X. Jiang, J. F. Hartwig, J. Am. Chem. Soc. 2019, 141, 13066–13073.
- 78Q. Zhang, H. Yu, L. Shen, T. Tang, D. Dong, W. Chai, W. Zi, J. Am. Chem. Soc. 2019, 141, 14554–14559.
- 79S.-M. Xu, L. Wei, C. Shen, L. Xiao, H.-Y. Tao, C.-J. Wang, Nat. Commun. 2019, 10, 5553.
- 80M. Zhu, Q. Zhang, W. Zi, Angew. Chem. Int. Ed. 2021, 60, 6545–6552.
- 81S.-Q. Yang, Y.-F. Wang, W.-C. Zhao, G.-Q. Lin, Z.-T. He, J. Am. Chem. Soc. 2021, 143, 7285–7291.
- 82Y. Xu, H. Wang, Z. Yang, Y. Zhou, Y. Liu, X. Feng, Chem 2022, 8, 2011–2022.
- 83T. P. Le, S. Tanaka, M. Yoshimura, K. Sato, M. Kitamura, Nat. Commun. 2022, 13, 5876.
- 84Z. He, L. Peng, C. Guo, Nat. Synth. 2022, 1, 393–400.
- 85C. Fu, L. He, X. Chang, X. Cheng, Z.-F. Wang, Z. Zhang, V. A. Larionov, X.-Q. Dong, C.-J. Wang, Angew. Chem. Int. Ed. 2024, 63, e202315325.
- 86M.-Q. Tang, Z.-J. Yang, A.-J. Han, Z.-T. He, Angew. Chem. Int. Ed. 2025, 64, e202413428.
- 87B. Qing, Z. Yang, Z. Wu, Z. Zhang, Y. Zhou, X. Yan, Y. Liu, X. Feng, J. Am. Chem. Soc. 2025, 147, 7729–7740.
- 88A. Changotra, B. Bhaskararao, C. M. Hadad, R. B. Sunoj, J. Am. Chem. Soc. 2020, 142, 9612–9624.
- 89B. Li, H. Xu, Y. Dang, K. N. Houk, J. Am. Chem. Soc. 2022, 144, 1971–1985.
- 90B. Li, H. Xu, Y. Dang, Acc. Chem. Res. 2023, 56, 3260–3270.
- 91F. Gao, J. Li, T. Ahmad, Y. Luo, Z. Zhang, Q. Yuan, X. Huo, T. Song, W. Zhang, Sci. China Chem. 2022, 65, 1968–1977.
- 92Q. Wang, Q. Gu, S.-L. You, Angew. Chem. Int. Ed. 2019, 58, 6818–6825.
- 93X. Xiao, B. Zhao, Acc. Chem. Res. 2023, 56, 1097–1117.
- 94W. Wen, Q.-X. Guo, Acc. Chem. Res. 2024, 57, 776–794.
- 95J. Chen, X. Gong, J. Li, Y. Li, J. Ma, C. Hou, G. Zhao, W. Yuan, B. Zhao, Science 2018, 360, 1438–1442.
- 96W. Wen, L. Chen, M.-J. Luo, Y. Zhang, Y.-C. Chen, Q. Ouyang, Q.-X. Guo, J. Am. Chem. Soc. 2018, 140, 9774–9780.
- 97L. Chen, M.-J. Luo, F. Zhu, W. Wen, Q.-X. Guo, J. Am. Chem. Soc. 2019, 141, 5159–5163.
- 98A. Cheng, L. Zhang, Q. Zhou, T. Liu, J. Cao, G. Zhao, K. Zhang, G. Song, B. Zhao, Angew. Chem. Int. Ed. 2021, 60, 20166–20172.
- 99J. Ma, Q. Zhou, G. Song, Y. Song, G. Zhao, K. Ding, B. Zhao, Angew. Chem. Int. Ed. 2021, 60, 10588–10592.
- 100X. Zhong, Z. Zhong, Z. Wu, Z. Ye, Y. Feng, S. Dong, X. Liu, Q. Peng, X. Feng, Chem. Sci 2021, 12, 4353–4360.
- 101J. Ma, B. Gao, G. Song, R. Zhang, Q. Wang, Z. Ye, W.-W. Chen, B. Zhao, Angew. Chem. Int. Ed. 2022, 61, e202200850.
- 102P. Ji, X. Liu, J. Xu, X. Zhang, J. Guo, W.-W. Chen, B. Zhao, Angew. Chem. Int. Ed. 2022, 61, e202206111.
- 103F. Zhu, C.-X. Li, Z.-L. Wu, T. Cai, W. Wen, Q.-X. Guo, Nat. Commun. 2022, 13, 7290.
- 104J.-H. Liu, W. Wen, J. Liao, Q.-W. Shen, Y. Lin, Z.-L. Wu, T. Cai, Q.-X. Guo, Nat. Commun. 2022, 13, 2509.
- 105R. Zhang, J. Xu, S. Liu, S. Si, J. Chen, L. Wang, W.-W. Chen, B. Zhao, J. Am. Chem. Soc. 2024, 146, 25927–25933.
- 106Y. Ma, J. Li, J. Ye, D. Liu, W. Zhang, Chem. Commun. 2018, 54, 13571–13574.
- 107Y. Xu, D. Liu, Y. Deng, Y. Zhou, W. Zhang, Angew. Chem. Int. Ed. 2021, 60, 23602–23607.
- 108Y. Xu, Y. Luo, J. Ye, Y. Deng, D. Liu, W. Zhang, J. Am. Chem. Soc. 2022, 144, 20078–20089.
- 109S. Xu, W. Xu, S. Dong, D. Liu, W. Zhang, Chem. - Eur. J. 2024, 30, e202400978.
- 110R. Narayan, M. Potowski, Z.-J. Jia, A. P. Antonchick, H. Waldmann, Acc. Chem. Res. 2014, 47, 1296–1310.
- 111T. Hashimoto, K. Maruoka, Chem. Rev. 2015, 115, 5366–5412.
- 112L. Wei, X. Chang, C.-J. Wang, Acc. Chem. Res. 2020, 53, 1084–1100.
- 113B. List, Chem. Commun. 2006, 819–824.
- 114A. Erkkilä, I. Majander, P. M. Pihko, Chem. Rev. 2007, 107, 5416–5470.
- 115D. W. C. MacMillan, Nature 2008, 455, 304–308.
- 116L. Hong, W. Sun, D. Yang, G. Li, R. Wang, Chem. Rev. 2016, 116, 4006–4123.
- 117 Deposition number 2364281 (for (R,R)-3ca) and 2364284 (for 8) contain the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service.
- 118T. Lu, Q. Chen, J. Comput. Chem. 2022, 43, 539–555.
- 119T. Lu, Q. Chen, J. Mol. Model. 2020, 26, 315.