Photoacid-Induced Supramolecular Network Disassembly: A Systems Approach to Stimuli-Responsive Polymers
Marta Oggioni
Adolphe Merkle Institute (AMI), University of Fribourg, Chemin des Verdiers 4, Fribourg, 1700 Switzerland
Search for more papers by this authorLuca Bertossi
Adolphe Merkle Institute (AMI), University of Fribourg, Chemin des Verdiers 4, Fribourg, 1700 Switzerland
Search for more papers by this authorDr. Derek J. Kiebala
Adolphe Merkle Institute (AMI), University of Fribourg, Chemin des Verdiers 4, Fribourg, 1700 Switzerland
Department of Chemistry, University of Mainz, Duesbergweg 10–14, Mainz, 55128 Germany
Search for more papers by this authorAdelle Kirshner
Adolphe Merkle Institute (AMI), University of Fribourg, Chemin des Verdiers 4, Fribourg, 1700 Switzerland
Department of Chemistry and Biochemistry, California Polytechnic State University-San Luis Obispo, 1 Grand Avenue, San Luis Obispo, California, 93407 USA
Search for more papers by this authorDr. Andrea Dodero
Adolphe Merkle Institute (AMI), University of Fribourg, Chemin des Verdiers 4, Fribourg, 1700 Switzerland
Search for more papers by this authorCorresponding Author
Dr. Georges J. M. Formon
Adolphe Merkle Institute (AMI), University of Fribourg, Chemin des Verdiers 4, Fribourg, 1700 Switzerland
E-mail: [email protected]; [email protected]
Search for more papers by this authorCorresponding Author
Prof. Dr. Christoph Weder
Adolphe Merkle Institute (AMI), University of Fribourg, Chemin des Verdiers 4, Fribourg, 1700 Switzerland
E-mail: [email protected]; [email protected]
Search for more papers by this authorMarta Oggioni
Adolphe Merkle Institute (AMI), University of Fribourg, Chemin des Verdiers 4, Fribourg, 1700 Switzerland
Search for more papers by this authorLuca Bertossi
Adolphe Merkle Institute (AMI), University of Fribourg, Chemin des Verdiers 4, Fribourg, 1700 Switzerland
Search for more papers by this authorDr. Derek J. Kiebala
Adolphe Merkle Institute (AMI), University of Fribourg, Chemin des Verdiers 4, Fribourg, 1700 Switzerland
Department of Chemistry, University of Mainz, Duesbergweg 10–14, Mainz, 55128 Germany
Search for more papers by this authorAdelle Kirshner
Adolphe Merkle Institute (AMI), University of Fribourg, Chemin des Verdiers 4, Fribourg, 1700 Switzerland
Department of Chemistry and Biochemistry, California Polytechnic State University-San Luis Obispo, 1 Grand Avenue, San Luis Obispo, California, 93407 USA
Search for more papers by this authorDr. Andrea Dodero
Adolphe Merkle Institute (AMI), University of Fribourg, Chemin des Verdiers 4, Fribourg, 1700 Switzerland
Search for more papers by this authorCorresponding Author
Dr. Georges J. M. Formon
Adolphe Merkle Institute (AMI), University of Fribourg, Chemin des Verdiers 4, Fribourg, 1700 Switzerland
E-mail: [email protected]; [email protected]
Search for more papers by this authorCorresponding Author
Prof. Dr. Christoph Weder
Adolphe Merkle Institute (AMI), University of Fribourg, Chemin des Verdiers 4, Fribourg, 1700 Switzerland
E-mail: [email protected]; [email protected]
Search for more papers by this authorGraphical Abstract
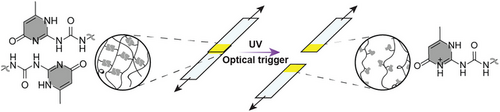
Light-responsive supramolecular (SM) materials are attractive for many applications but are difficult to achieve. Here, we report a systems approach that involves blending a hydrogen-bonded SM network with a photoacid generator. The resulting material displays significant softening upon UV irradiation and functions as an efficient debonding-on-demand adhesive.
Abstract
The introduction of supramolecular (SM) motifs allows the design of stimuli-responsive polymers whose physical properties can be changed by external triggers that affect the SM binding. However, using this approach to create light-responsive materials that can be switched under isothermal conditions proves to be challenging. Here, we report a material systems approach to achieve this. Thus, SM polymer networks, in which optically inert ureidopyrimidinone (UPy) groups assemble into reversible cross-links, are combined with a trigger molecule, the photoacid generator (PAG) 2-(4-methoxy-styryl)-4,6-bis(trichloromethyl)-1,3,5-triazine (MBTT). Model experiments with a fluorescent, self-reporting UPy motif elucidate the sequence of processes that result from optical triggering, namely acid generation, protonation of UPy groups, and ultimately dissociation of the SM cross-links. The optically stimulated transformation of gels and rubbery films into viscous liquids was quantitatively monitored by opto-rheological measurements, showing that the optical stimulation and resultant mechanical responses are intimately coupled. The potential usefulness of this approach to create materials with spatially graded mechanical characteristics and as the basis for debonding-on-demand (DOD) adhesives was explored in proof-of-concept experiments.
Conflict of Interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available for download at the Zenodo repository at 10.5281/zenodo.15050468.
Supporting Information
| Filename | Description |
|---|---|
| anie202506981-sup-0001-SuppMat.docx19.1 MB | Supporting Information |
| anie202506981-sup-0002-SupplementaryVideo1.mp416.8 MB | Supporting Information |
| anie202506981-sup-0003-SupplementaryVideo2.mp415.3 MB | Supporting Information |
| anie202506981-sup-0004-SupplementaryVideo3.mp428.6 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1K. Tashiro, K. Katayama, K. Tamaki, L. Pesce, N. Shimizu, H. Takagi, R. Haruki, M. J. Hollamby, G. M. Pavan, S. Yagai, Angew. Chem. Int. Ed. 2021, 60, 26986–26993.
- 2D. J. Lundberg, C. M. Brown, E. O. Bobylev, N. J. Oldenhuis, Y. S. Alfaraj, J. Zhao, I. Kevlishvili, H. J. Kulik, J. A. Johnson, Nat. Commun. 2024, 15, 3951.
- 3J. R. Kumpfer, S. J. Rowan, J. Am. Chem. Soc. 2011, 133, 12866–12874.
- 4N. E. Botterhuis, D. J. M. Van Beek, G. M. L. Van Gemert, A. W. Bosman, R. P. Sijbesma, J. Polym. Sci. A Polym. Chem. 2008, 46, 3877–3885.
- 5J. Zhang, C. Jiang, G. Deng, M. Luo, B. Ye, H. Zhang, M. Miao, T. Li, D. Zhang, Nat. Commun. 2024, 15, 4869.
- 6Z. Zhang, D. Lei, C. Zhang, Z. Wang, Y. Jin, W. Zhang, X. Liu, J. Sun, Adv. Mater. 2023, 35, 2208619.
- 7C. Fouquey, J. M. Lehn, A. M. Levelut, Adv. Mater. 1990, 2, 254–257.
- 8Z. Zhang, Z. Hu, J. Xing, Q. Li, Responsive Mater. 2024, 2, e20240009.
- 9T. Nakamura, Y. Takashima, A. Hashidzume, H. Yamaguchi, A. Harada, Nat. Commun. 2014, 5, 4622.
- 10A. C. Ferahian, D. K. Hohl, C. Weder, L. Montero de Espinosa, Macromol. Mater. Eng. 2019, 304, 1900161.
- 11W. Wang, Y. Xu, Y. Tang, Q. Li, Adv. Mater. 2025, 37, 2416122.
- 12H. Zhang, S. Yang, Z. Yang, D. Wang, J. Han, C. Li, C. Zhu, J. Xu, N. Zhao, ACS Appl. Mater. Interfaces 2021, 13, 4499–4507.
- 13X. Wang, J. Xu, Y. Zhang, T. Wang, Q. Wang, S. Li, Z. Yang, X. Zhang, Nat. Commun. 2023, 14, 4712.
- 14R. Suriano, L. Brambilla, M. Tommasini, S. Turri, Polym. Adv. Technol. 2018, 29, 2899–2908.
- 15D. K. Hohl, A. C. Ferahian, L. Montero De Espinosa, C. Weder, ACS Macro Lett. 2019, 8, 1484–1490.
- 16J. Guo, Y. Li, Y. Zhang, J. Ren, X. Yu, X. Cao, ACS Appl. Mater. Int. 2021, 13, 40079–40087.
- 17Y. Meng, Y. Liu, Z. Wan, Y. Huan, Q. Guo, D. Fan, X. Zhou, J. Liu, Y. Cao, X. Cao, Z. Gu, T. Qian, C. Yan, Chem. Eng. J. 2023, 453, 139967.
- 18C. Qin, Y. Feng, H. An, J. Han, C. Cao, W. Feng, ACS Appl. Mater. Int. 2017, 9, 4066–4073.
- 19P. Wang, C. Huang, Y. Xing, W. Fang, J. Ren, H. Yu, G. Wang, Langmuir 2019, 35, 1021–1031.
- 20Y. Q. Zou, B. Hu, L. Chen, T. Ji, M. Yang, C. A. Yang, Iranian Pol. J.(Eng. Ed.) 2021, 30, 47–56.
- 21R. Bai, H. Zhang, X. Yang, J. Zhao, Y. Wang, Z. Zhang, X. Yan, Polym. Chem. 2022, 13, 1253–1259.
- 22Y. Q. Jiang, K. Wu, Q. Zhang, K. Q. Li, Y. Y. Li, P. Y. Xin, W. W. Zhang, H. M. Guo, Chem. Commun. 2018, 54, 13821–13824.
- 23Y. Ding, C. Wang, Y. Ma, L. Zhu, B. Lu, Y. Wang, J. Wang, T. Chen, C. M. Dong, Y. Yao, Acta Biomater. 2022, 143, 381–391.
- 24C. Heinzmann, U. Salz, N. Moszner, G. L. Fiore, C. Weder, ACS Appl. Mater. Interfaces 2015, 7, 13395–13404.
- 25S. Burattini, H. M. Colquhoun, B. W. Greenland, W. Hayes, Faraday Discuss. 2009, 143, 251–264.
- 26D. J. Phillips, M. Wilde, F. Greco, M. I. Gibson, Biomacromol. 2015, 16, 3256–3264.
- 27D. J. Phillips, I. Prokes, G. L. Davies, M. I. Gibson, ACS Macro Lett. 2014, 3, 1225–1229.
- 28C. Heinzmann, S. Coulibaly, A. Roulin, G. L. Fiore, C. Weder, ACS Appl Mater. Inter. 2014, 6, 4713–4719.
- 29D. W. R. Balkenende, C. A. Monnier, G. L. Fiore, C. Weder, Nat. Commun. 2016, 7, 10995.
- 30J. Verjans, R. Hoogenboom, Prog. Polym. Sci. 2023, 142, 101689.
- 31S. H. M. Söntjens, R. P. Sijbesma, M. H. P. Van Genderen, E. W. Meijer, J. Am. Chem. Soc. 2000, 122, 7487–7493.
- 32B. Tang, M. Pauls, C. Bannwarth, S. Hecht, J. Am. Chem. Soc. 2024, 146, 45–50.
- 33S. T. Han, H. Y. Duan, T. G. Zhan, X. B. Hu, L. C. Kong, K. D.a Zhang, Chinese Chem. Lett. 2023, 34, 107639.
- 34J. J. B. van der Tol, T. A. P. Engels, R. Cardinaels, G. Vantomme, E. W. Meijer, F. Eisenreich, Adv. Funct. Mater. 2023, 33, 2301246.
- 35S. Ito, H. Akiyama, R. Sekizawa, M. Mori, T. Fukata, M. Yoshida, H. Kihara, J. Polym. Sci. A Polym. Chem. 2019, 57, 806–813.
- 36Y. M. Tseng, A. Narayanan, K. Mishra, X. Liu, A. Joy, ACS Appl. Mater. Int. 2021, 13, 29048–29057.
- 37K. Yamauchi, J. R. Lizotte, T. E. Long, Macromol 2003, 36, 1083–1088.
- 38T. F. A. De Greef, M. J. Kade, K. E. Feldman, E. J. Kramer, C. J. Hawker, E. W. Meijer, J. Polym. Sci. A Polym. Chem. 2011, 49, 4253–4260.
- 39L. Thomson, R. Schweins, E. R. Draper, D. J. Adams, Macromol. Rapid Commun. 2020, 41, 2000093.
- 40D. J. Kiebala, A. Dodero, C. Weder, S. Schrettl, Angew. Chem. Int. Ed. 2024, 63, e202405922.
- 41X. Z. Niu, R. D. Pepel, R. Paniego, J. A. Field, J. Chorover, L. Abrell, A. E. Sáez, R. Sierra-Alvarez, J Photochem. Photobiol. A Chem. 2021, 416, 113324.
- 42C. J. Martin, G. Rapenne, T. Nakashima, T. Kawai, J. Photochem. Photobiol. C: Photochem. Rev. 2018, 34, 41–51.
- 43X. Peng, J. Zhang, Z. H. Stachurski, M. M. Banaszak Holl, P. Xiao, ACS Appl. Mater. Int. 2021, 13, 46033.
- 44G. Pohlers, J. C. Scaiano, Chem. Mater. 1997, 9, 1353–1361.
- 45H. Lai, D. Zhu, P. Xiao, Macromol. Chem. Phys. 2019, 220, 1900315.
- 46J. Zhang, P. Xiao, F. Morlet-Savary, B. Graff, J. P. Fouassier, J. Lalevée, Polym. Chem. 2014, 5, 6019–6026.
- 47G. Pohlers, J. C. Scaiano, E. Step, R. Sinta, J. Am. Chem. Soc. 1999, 121, 6167–6175.
- 48E. J. Foster, E. B. Berda, E. W. Meijer, J. Am. Chem. Soc. 2009, 131, 6964–6966.
- 49T. Iwata, K. Kinashi, H. N. Doan, P. P. Vo, W. Sakai, N. Tsutsumi, ACS Omega 2019, 4, 9946–9951.
- 50J. Cui, D. Wang, K. Koynov, A. del Campo, ChemPhysChem 2013, 14, 2932–2938.
- 51H. Xu, X.-M. Xie, Chin. Chem. Lett. 2021, 32, 521–524.
- 52I. Onori, J. A. Berrocal, C. Weder, Polymer 2024, 298, 126886.
- 53A. M. Api, D. Belsito, D. Botelho, M. Bruze, G. A. Burton, J. Buschmann, M. A. Cancellieri, M. L. Dagli, M. Date, W. Dekant, C. Deodhar, A. D. Fryer, L. Jones, K. Joshi, M. Kumar, A. Lapczynski, M. Lavelle, I. Lee, D. C. Liebler, H. Moustakas, M. Na, T. M. Penning, G. Ritacco, J. Romine, N. Sadekar, T. W. Schultz, D. Selechnik, F. Siddiqi, I. G. Sipes, G. Sullivan, et al., Food Chem. Toxicol. 2022, 159, 112680.
- 54W. Wang, Z. An, Z. Wang, S. Wang, Chem. Eur. J. 2024, 30, e202304349.
- 55S. Sadeghzade, J. Cao, D. Zhang, P. Dong, J. Hu, A. Es'haghioskui, H. Yuan, Eur. Polym. J. 2023, 197, 112337.