Remote Spin-Center Shift Enables Activation of Distal Benzylic C─O and C─N Bonds
Li-Wen Hui
State Key Laboratory of Precision and Intelligent Chemistry, Anhui Provincial Key Laboratory of Biomass Chemistry, Department of Chemistry, University of Science and Technology of China, 96 Jinzhai Road, Hefei, Anhui, 230026 China
These authors contributed equally to this work.
Search for more papers by this authorYee Lin Phang
State Key Laboratory of Precision and Intelligent Chemistry, Anhui Provincial Key Laboratory of Biomass Chemistry, Department of Chemistry, University of Science and Technology of China, 96 Jinzhai Road, Hefei, Anhui, 230026 China
These authors contributed equally to this work.
Search for more papers by this authorChen-Yang Ye
State Key Laboratory of Precision and Intelligent Chemistry, Anhui Provincial Key Laboratory of Biomass Chemistry, Department of Chemistry, University of Science and Technology of China, 96 Jinzhai Road, Hefei, Anhui, 230026 China
These authors contributed equally to this work.
Search for more papers by this authorJin-Yu Lai
Institute of Advanced Technology, University of Science and Technology of China, 96 Jinzhai Road, Hefei, Anhui, 230026 China
Search for more papers by this authorFeng-Lian Zhang
State Key Laboratory of Precision and Intelligent Chemistry, Anhui Provincial Key Laboratory of Biomass Chemistry, Department of Chemistry, University of Science and Technology of China, 96 Jinzhai Road, Hefei, Anhui, 230026 China
Search for more papers by this authorCorresponding Author
Yao Fu
State Key Laboratory of Precision and Intelligent Chemistry, Anhui Provincial Key Laboratory of Biomass Chemistry, Department of Chemistry, University of Science and Technology of China, 96 Jinzhai Road, Hefei, Anhui, 230026 China
E-mail: [email protected]; [email protected]
Search for more papers by this authorCorresponding Author
Yi-Feng Wang
State Key Laboratory of Precision and Intelligent Chemistry, Anhui Provincial Key Laboratory of Biomass Chemistry, Department of Chemistry, University of Science and Technology of China, 96 Jinzhai Road, Hefei, Anhui, 230026 China
E-mail: [email protected]; [email protected]
Search for more papers by this authorLi-Wen Hui
State Key Laboratory of Precision and Intelligent Chemistry, Anhui Provincial Key Laboratory of Biomass Chemistry, Department of Chemistry, University of Science and Technology of China, 96 Jinzhai Road, Hefei, Anhui, 230026 China
These authors contributed equally to this work.
Search for more papers by this authorYee Lin Phang
State Key Laboratory of Precision and Intelligent Chemistry, Anhui Provincial Key Laboratory of Biomass Chemistry, Department of Chemistry, University of Science and Technology of China, 96 Jinzhai Road, Hefei, Anhui, 230026 China
These authors contributed equally to this work.
Search for more papers by this authorChen-Yang Ye
State Key Laboratory of Precision and Intelligent Chemistry, Anhui Provincial Key Laboratory of Biomass Chemistry, Department of Chemistry, University of Science and Technology of China, 96 Jinzhai Road, Hefei, Anhui, 230026 China
These authors contributed equally to this work.
Search for more papers by this authorJin-Yu Lai
Institute of Advanced Technology, University of Science and Technology of China, 96 Jinzhai Road, Hefei, Anhui, 230026 China
Search for more papers by this authorFeng-Lian Zhang
State Key Laboratory of Precision and Intelligent Chemistry, Anhui Provincial Key Laboratory of Biomass Chemistry, Department of Chemistry, University of Science and Technology of China, 96 Jinzhai Road, Hefei, Anhui, 230026 China
Search for more papers by this authorCorresponding Author
Yao Fu
State Key Laboratory of Precision and Intelligent Chemistry, Anhui Provincial Key Laboratory of Biomass Chemistry, Department of Chemistry, University of Science and Technology of China, 96 Jinzhai Road, Hefei, Anhui, 230026 China
E-mail: [email protected]; [email protected]
Search for more papers by this authorCorresponding Author
Yi-Feng Wang
State Key Laboratory of Precision and Intelligent Chemistry, Anhui Provincial Key Laboratory of Biomass Chemistry, Department of Chemistry, University of Science and Technology of China, 96 Jinzhai Road, Hefei, Anhui, 230026 China
E-mail: [email protected]; [email protected]
Search for more papers by this authorGraphical Abstract
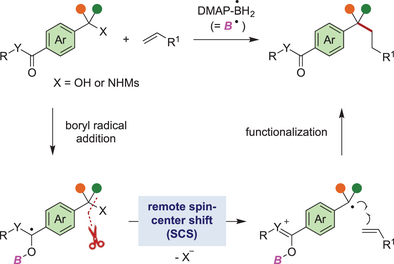
A remote spin-center shift process that involves 1,n-radical translocation followed by elimination of a leaving group has been developed to enable distal benzylic C─O and C─N bond activation. By this process, a 4-dimethylaminopyridine (DMAP)-boryl radical promoted radical deoxygenative and deaminative functionalization of free benzylic alcohols and simple benzylic amines to afford a wide range of alkylated products.
Abstract
A spin-center shift (SCS) is a radical process that commonly involves a 1,2-radical shift along with the elimination of an adjacent leaving group by a two-electron ionic movement. The conventional SCS process is largely limited to 1,2-radical translocation, while a remote SCS event involving 1,n-radical translocation over a greater distance to enable distal bond functionalization remains largely underexplored. Herein, we report the boryl radical-promoted distal deoxygenation and deamination of free benzylic alcohols and simple benzylic amines, respectively, through a remote SCS event. The reaction was initiated by the addition of a 4-dimethylaminopyridine (DMAP)-boryl radical to the carbonyl oxygen atom of a benzoate or benzamide. Then, radical translocation took place across the aromatic ring to promote benzylic C─O or C─N bond cleavage. The resulting radical intermediate subsequently coupled with various alkenes to afford a wide range of alkylated products. The proposed mechanistic pathway was supported by experimental investigations.
Conflict of Interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.
Supporting Information
| Filename | Description |
|---|---|
| anie202506771-sup-0001-SuppMat.pdf21.2 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1P. Wessig, O. Muehling, S.-C. Shift, Eur. J. Org. Chem. 2007, 2007, 2219–2232.
- 2F.-L. Zhang, B. Li, K. N. Houk, Y.-F. Wang, JACS Au 2022, 2, 1032–1042.
- 3B. Matsuo, A. Granados, J. Majhi, M. Sharique, G. Levitre, G. A. Molander, ACS Org. Inorg. Au 2022, 2, 435–454.
- 4E. D. Nacsa, D. W. C. MacMillan, J. Am. Chem. Soc. 2018, 140, 3322–3330.
- 5T. Henkel, R. M. Brunne, H. Müller, F. Reichel, Angew. Chem. Int. Ed. 38, 643–647.
10.1002/(SICI)1521-3773(19990301)38:5<643::AID-ANIE643>3.0.CO;2-G PubMed Web of Science® Google Scholar
- 6P. Ertl, T. Schuhmann, J. Nat. Prod. 2019, 82, 1258–1263.
- 7Y. Zabolotna, D. M. Volochnyuk, S. V. Ryabukhin, D. Horvath, K. S. Gavrilenko, G. Marcou, Y. S. Moroz, O. Oksiuta, A. Varnek, J. Chem. Inf. Model. 2022, 62, 2171–2185.
- 8Y.-R. Luo, in Comprehensive Handbook of Chemical Bond Energies, 1st ed., CRC press, Boca Raton 2007.
10.1201/9781420007282 Google Scholar
- 9K. Anwar, K. Merkens, F. J. Aguilar Troyano, A. Gómez-Suárez, Eur. J. Org. Chem. 2022, 2022, e202200330.
- 10F. Mo, D. Qiu, L. Zhang, J. Wang, Chem. Rev. 2021, 121, 5741–5829.
- 11X. Chen, N.-Z. Wang, Y.-M. Cheng, X. Kong, Z.-Y. Cao, Synthesis 2023, 55, 2833–2842.
- 12Y. Gao, S. Jiang, N.-D. Mao, H. Xiang, J.-L. Duan, X.-Y. Ye, L.-W. Wang, Y. Ye, T. Xie, Top. Curr. Chem. 2022, 380, 25.
- 13T. Mandal, S. Mallick, M. Islam, S. De Sarkar, ACS Catal.2024, 14, 13451–13496
- 14M. Roy, B. Sardar, I. Mallick, D. Srimani, Beilstein J. Org. Chem. 2024, 20, 1348–1375.
- 15K. J. Berger, J. L. Driscoll, M. Yuan, B. D. Dherange, O. Gutierrez, M. D. Levin, J. Am. Chem. Soc. 2021, 143, 17366–17373.
- 16L. Cheng, Q. Lin, Y. Chen, H. Gong, Synthesis 2022, 54, 4426–4446.
- 17X. B. Pang, X.-Z. Shu, Chin. J. Chem. 2023, 41, 1637–1652.
- 18H. Xie, S. Wang, X.-Z. Shu, C.-O. H. Bond, J. Am. Chem. Soc. 2024, 146, 32269–32275.
- 19W.-D. Li, Y. Wu, S.-J. Li, Y.-Q. Jiang, Y.-L. Li, Y. Lan, J.-B. Xia, J. Am. Chem. Soc. 2022, 144, 8551–8559.
- 20C. Bandari, K. M. Nicholas, J. Org. Chem. 2020, 85, 3320–3327.
- 21C. Boucher-Jacobs, P. Liu, K. M. Nicholas, Organometallics 2018, 37, 2468–2480.
- 22E. Steffensmeier, K. M. Nicholas, Chem. Commun. 2018, 54, 790–793.
- 23J. G. Uranga, A. F. Chiosso, A. N. Santiago, RSC Adv. 2013, 3, 11493.
- 24J. Jin, D. W. C. MacMillan, Nature 2015, 525, 87–90.
- 25J. Dong, Z. Wang, X. Wang, H. Song, Y. Liu, Q. Wang, Sci. Adv. 2019, 5, eaax9955.
- 26Y. Masuda, H. Tsuda, M. Murakami, Angew. Chem. Int. Ed. 2020, 59, 2755–2759.
- 27Z.-S. Wang, Y.-B. Chen, H.-W. Zhang, Z. Sun, C. Zhu, L.-W. Ye, J. Am. Chem. Soc. 2020, 142, 3636–3644.
- 28H. M. Carder, C. E. Suh, A. E. Wendlandt, J. Am. Chem. Soc. 2021, 143, 13798–13805.
- 29T. T. Simur, T.-Y. Peng, Y.-F. Wang, X.-W. Wu, F.-L. Zhang, Org. Lett. 2023, 25, 2270–2274.
- 30W. Yan, Z. Qu, F. Zhao, G.-J. Deng, G.-J. Mao, H. Huang, Adv. Synth. Catal. 2023, 365, 612–617.
- 31E. K. Taskinen, D. Kolb, M. Morgenstern, B. König, Chem.-Eur. J. 2024, e202404200.
- 32Z. Wang, X. Ji, T. Han, G. J. Deng, H. Huang, Adv. Synth. Catal. 2019, 361, 5643–5647.
- 33W.-Y. Wang, J.-J. Wang, J.-B. Pan, L.-J. Xiao, Q.-L. Zhou, Chem 2024, 10, 3667–3677.
- 34K. Chen, J. Schwarz, T. A. Karl, A. Chatterjee, B. König, Chem. Commun. 2019, 55, 13144–13147.
- 35Q. Zhu, D. G. Nocera, ACS Catal. 2021, 11, 14181–14187.
- 36M. H. Larraufie, R. Pellet, L. Fensterbank, J. P. Goddard, E. Lacôte, M. Malacria, C. Ollivier, Angew. Chem. Int. Ed. 2011, 50, 4463–4466.
- 37Y.-J. Yu, F.-L. Zhang, T.-Y. Peng, C.-L. Wang, J. Cheng, C. Chen, K. N. Houk, Y.-F. Wang, Science 2021, 371, 1232–1240.
- 38Q. Zhao, B. Li, X. Zhou, Z. Wang, F.-L. Zhang, Y. Li, X. Zhou, Y. Fu, Y.-F. Wang, J. Am. Chem. Soc. 2022, 144, 15275–15285.
- 39T. T. Simur, F. W. Dagnaw, Y.-J. Yu, F.-L. Zhang, Y.-F. Wang, 4 Chin. J. Chem. 2022, 40, 577–581.
- 40T.-Y. Peng, Z.-Y. Xu, F.-L. Zhang, B. Li, W.-P. Xu, Y. Fu, Y.-F. Wang, Angew. Chem. Int. Ed. 2022, 61, e202201329.
- 41T.-Y. Peng, F.-L. Zhang, Y.-F. Wang, Acc. Chem. Res. 2023, 56, 169–186.
- 42W. Dai, T. R. McFadden, D. P. Curran, H. A. Früchtl, J. C. Walton, J. Am. Chem. Soc. 2018, 140, 15868–15875.
- 43W. Dai, D. P. Curran, J. C. Walton, J. Org. Chem. 2019, 84, 2102–2111.
- 44D. A. Bolt, D. P. Curran, Adv. Synth. Catal. 2020, 362, 2238–2244.
- 45J. C. Walton, W. Dai, D. P. Curran, J. Org. Chem. 2020, 85, 4248–4255.
- 46J. Lalevée, N. Blanchard, M. A. Tehfe, A. C. Chany, J. P. Fouassier, Chem.-Eur. J. 2010, 16, 12920–12927.
- 47F. Barth, F. Achrainer, A. M. Pütz, H. Zipse, Chem.-Eur. J. 2017, 23, 13455–13464.
- 48B. P. Roberts, Chem. Soc. Rev. 1999, 28, 25–35.
- 49F. Zhang, Y. Li, X. Zhou, Q. Zhao, X. Li, F.-L. Zhang, Y.-F. Wang, X. Zhou, Chem.-Eur. J. 2024, e202403949.
- 50X. Pan, E. Lacôte, J. Lalevée, D. P. Curran, J. Am. Chem. Soc. 2012, 134, 5669–5674.
- 51P. Veeraraghavan Ramachandran, A. S. Kulkarni, Y. Zhao, J. Mei, Chem. Commun. 2016, 52, 11885–11888.
- 52M. M. Brahmi, J. Monot, M. Desage-El Murr, D. P. Curran, L. Fensterbank, E. Lacôte, M. Malacria, J. Org. Chem. 2010, 75, 6983–6985.
- 53N. P. Taylor, J. A. Gonzalez, G. S. Nichol, A. García-Domínguez, A. G. Leach, G. C. Lloyd-Jones, A. Lewis, J. Org. Chem. 2022, 87, 721–729.
- 54D. Tsvelikhovsky, S. L. Buchwald, J. Am. Chem. Soc. 2010, 132, 14048–14051.
- 55L. Zhang, Z. Huang, J. Am. Chem. Soc. 2015, 137, 15600–15603.
- 56Y.-J. Xiang, S. Liu, J. Zhou, J.-H. Lin, X. Yao, J.-C. Xiao, J. Org. Chem. 2023, 88, 4818–4828.
- 57G. Amato, S. Runyon, V. Vasukuttan, A. M. Decker, E. A. Gay, L. Laudermilk, R. Maitra, Bioorg. Med. Chem. Lett. 2023, 93, 129430.
- 58R. Narobe, K. Murugesan, C. Haag, T. E. Schirmer, B. König, Chem. Commun. 2022, 58, 8778–8781.
- 59F. Kloss, T. Neuwirth, V. G. Haensch, C. Hertweck, Angew. Chem. Int. Ed. 2018, 57, 14476–14481.
- 60H. Hu, Z. Shi, X. Guo, F.-H. Zhang, Z. Wang, J. Am. Chem. Soc. 2024, 146, 5316–5323.
- 61X. Wu, L. Hu, Tetrahedron Lett. 2005, 46, 8401–8405.