Modification of [2.2]Paracyclophanes via Cobalt/Salox-Catalyzed Enantioselective Electrooxidative or Photoredox C─H Acyloxylation and Alkoxylation
Fan-Rui Huang
Department of Chemistry, Zhejiang University, Hangzhou, 310027 China
Both authors contributed equally to this work.
Search for more papers by this authorMing-Ya Teng
Department of Chemistry, Zhejiang University, Hangzhou, 310027 China
Both authors contributed equally to this work.
Search for more papers by this authorHui Qiu
Department of Chemistry, Zhejiang University, Hangzhou, 310027 China
Search for more papers by this authorCorresponding Author
Qi-Jun Yao
Department of Chemistry, Zhejiang University, Hangzhou, 310027 China
E-mail: [email protected]; [email protected]
Search for more papers by this authorCorresponding Author
Bing-Feng Shi
Department of Chemistry, Zhejiang University, Hangzhou, 310027 China
School of Chemistry and Chemical Engineering, Henan Normal University, Xinxiang, 453007 China
College of Material Chemistry and Chemical Engineering, Key Laboratory of Organosilicon Chemistry and Material Technology, Ministry of Education, Hangzhou Normal University, Hangzhou, 311121 China
E-mail: [email protected]; [email protected]
Search for more papers by this authorFan-Rui Huang
Department of Chemistry, Zhejiang University, Hangzhou, 310027 China
Both authors contributed equally to this work.
Search for more papers by this authorMing-Ya Teng
Department of Chemistry, Zhejiang University, Hangzhou, 310027 China
Both authors contributed equally to this work.
Search for more papers by this authorHui Qiu
Department of Chemistry, Zhejiang University, Hangzhou, 310027 China
Search for more papers by this authorCorresponding Author
Qi-Jun Yao
Department of Chemistry, Zhejiang University, Hangzhou, 310027 China
E-mail: [email protected]; [email protected]
Search for more papers by this authorCorresponding Author
Bing-Feng Shi
Department of Chemistry, Zhejiang University, Hangzhou, 310027 China
School of Chemistry and Chemical Engineering, Henan Normal University, Xinxiang, 453007 China
College of Material Chemistry and Chemical Engineering, Key Laboratory of Organosilicon Chemistry and Material Technology, Ministry of Education, Hangzhou Normal University, Hangzhou, 311121 China
E-mail: [email protected]; [email protected]
Search for more papers by this authorHomepage: http://mypage.zju.edu.cn/en/bfshi
Graphical Abstract
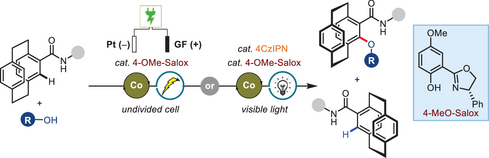
An efficient protocol with an excellent s-factor for the preparation of chiral [2.2]paracyclophanes through cobalt-catalyzed enantioselective C─H oxygenation was developed. This approach leverages traceless electrons or oxygen to replace traditional stoichiometric metal oxidants, facilitating the reaction under mild conditions with broad scope.
Abstract
Chiral [2.2]paracyclophanes (PCPs) have widespread application in asymmetric catalysis and materials science. However, enantioselective C─H activation of PCPs remains elusive and challenging due to steric hindrance, which differs significantly from conventional aryl C─H bonds. Herein, we present a cobalt/Salox-catalyzed enantioselective dehydrogenative C─H acyloxylation and alkoxylation of racemic PCPs with carboxylic acids and alcohols under electrooxidative or photoredox conditions. This innovative approach leverages traceless electrons or oxygen to replace traditional stoichiometric metal oxidants, allowing the reaction to proceed under mild conditions. The method enables the efficient synthesis of oxygenated optically enriched PCPs, achieving yields of up to 50% with 99% ee, as well as up to 49% yields and 99% ee for the recovered starting materials, resulting in exceptional s-factors of up to 1057. The reaction exhibits a broad scope, accommodating a diverse array of carboxylic acids, including complicated natural products and pharmaceutical molecules. This strategy not only provides an efficient route for synthesizing optically enriched PCP compounds but also highlights the potential of electrooxidative and photoredox methodologies in asymmetric C─H activation reactions.
Conflict of Interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the Supporting Information of this article.
Supporting Information
| Filename | Description |
|---|---|
| anie202506465-sup-0001-SuppMat.pdf15.4 MB | Supporting information |
| anie202506465-sup-0002-SuppMat.zip1.8 MB | Supporting information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1C. J. Brown, A. C. Farthing, Nature 1949, 164, 915–916.
- 2D. J. Cram, H. Steinberg, J. Am. Chem. Soc. 1951, 73, 5691–5704.
- 3R. Gleiter, H. Hopf, Modern Cyclophane Chemistry, 1st ed., Wiley-VCH, Weinheim, 2004.
10.1002/3527603964 Google Scholar
- 4 For reviews on the application of planar chiral PCPs, see: R. López, C. Palomo, Angew. Chem. Int. Ed. 2022, 61, e202113504.
- 5Z. Hassan, E. Spuling, D M. Knoll, S. Bräse, Angew. Chem. Int. Ed. 2020, 59, 2156–2170.
- 6Z. Hassan, E. Spuling, D M. Knoll, J. Lahann, S. Bräse, Chem. Soc. Rev. 2018, 47, 6947–6963.
- 7M. Christmann, S. Brase, Asymmetric Synthesis, The Essentials, Wiley-VCH, Weinheim, 2006.
- 8T. G. Driver, J. R. Harris, K. A. Woerpel, J. Am. Chem. Soc. 2007, 129, 3836–3837.
- 9M.-W. Chen, B. Wu, Z. Liu, Y.-G. Zhou, Acc. Chem. Res. 2023, 56, 2096–2109.
- 10M. Cakici, Z.-G. Gu, M. Nieger, J. Bürck, L. Heinke, S. Bräse, Chem. Commun. 2015, 51, 4796–4798.
- 11Y. Morisaki, M. Gon, T. Sasamori, N. Tokitoh, Y. Chujo, J. Am. Chem. Soc. 2014, 136, 3350–3353.
- 12K. Schlotter, F. Boeckler, H. Hübner, P. Gmeiner, J. Med. Chem.2006, 49, 3628–3635.
- 13S. Felder, S. Wu, J. Brom, L. Micouin, E. Benedetti, Chirality 2021, 33, 506–527.
- 14C. J. Friedmann, S. Ay, S. Bräse, J. Org. Chem. 2010, 75, 4612–4614.
- 15V. Rozenberg, N. Dubrovina, E. Sergeeva, D. Antonov, R. J. Seacome, Beilstein J. Org. Chem. 2009, 5, 1.
- 16R. Parmar, M. P. Coles, P. B. Hitchcock, G. J. Rowlands, Synthesis 2010, 24, 4177–4187.
- 17A. Cipiciani, F. Bellezza, F. Fringuelli, M. G. Silvestrini, Tetrahedron: Asymmetry 2001, 12, 2277–2281.
- 18P. Dorizon, C. Martin, J.-C. Daran, J.-C. Fiaud, H.-B. Kagan, Tetrahedron: Asymmetry 2001, 12, 2625–2630.
- 19C. Zippel, Z. Hassan, A. Q. Parsa, J. Hohmann, S. Bräse, Adv. Synth. Catal. 2021, 363, 2861–2865.
- 20K. Akagawa, N. Nishi, I. Yoshikawa, K. Kudo, Eur. J. Org. Chem. 2015, 2015, 5055–5059.
- 21Y. Zhao, H. Wang, B. Wu, Y.-G. Zhou, Org. Chem. Front. 2019, 6, 3956–3960.
- 22Q. Liu, K. Teng, Y. Zhang, Y. Lv, Y. R. Chi, Z. Jin, Angew. Chem. Int. Ed. 2024, 63, e202406386.
- 23S. Yu, H. Bao, D. Zhang, X. Yang, Nat. Commun. 2023, 14, 5239.
- 24X. Zhang, Y. Zhou, Z.-X. Yu, C.-H. Tung, Z. Xu, Angew. Chem. Int. Ed. 2025, 64, e202420667.
- 25M.-L. Delcourt, S. Felder, E. Benedetti, L. Micouin, ACS Catal. 2018, 8, 6612–6616.
- 26V. Dočekal, F. Koucký, I. Císařová, J. Veselý, Nat. Commun. 2024, 15, 3090.
- 27D. Ly, J. Bacsa, H. M. L. Davies, ACS Catal. 2024, 14, 6423–6431.
- 28D. Chen, Y. Zhou, C.-H. Tung, Z.-X. Yu, Z. Xu, CCS Chem. 2025, DOI: 10.31635/ccschem.024.202404067.
- 29 For selected reviews on enantioselective C–H activation, see: S.-L. You, Asymmetric Functionalization of C–H Bonds. RSC, Cambridge, U.K., 2015.
- 30C. G. Newton, S.-G. Wang, C. C. Oliveira, N. Cramer, Chem. Rev. 2017, 117, 8908-8976.
- 31D.-W. Gao, Q. Gu, C. Zheng, S.-L. You, Acc. Chem. Res. 2017, 50, 351–365.
- 32T. G. Saint-Denis, R.-Y. Zhu, G. Chen, Q.-F. Wu, J.-Q. Yu, Science 2018, 359, eaao4798.
- 33G. Liao, T. Zhang, Z.-K. Lin, B.-F. Shi, Angew. Chem. Int. Ed. 2020, 59, 19773-19786.
- 34T. Yoshino, S. Satake, S. Matsunaga, Chem. - Eur. J. 2020, 26, 7346–7357.
- 35A. Kumar, A. Kumar, V. Krishnan, ACS Catal. 2020, 10, 10253–10315.
- 36O. Vyhivskyi, A. Kudashev, T. Miyakoshi, O. Baudoin, Chem. - Eur. J. 2021, 27, 1231–1257.
- 37C.-X. Liu, W.-W. Zhang, S.-Y. Yin, Q. Gu, S.-L. You, J. Am. Chem. Soc. 2021, 143, 14025–14040.
- 38X. Cong, L. Huang, Z. Hou, Tetrahedron 2023, 135, 133323.
- 39H. Liang, J. Wang, Chem. - Eur. J. 2023, 29, e202202461.
- 40P. Lennartz, G. Raabe, C. Bolm, Adv. Synth. Catal. 2012, 354, 3237–3249.
- 41J. J. P. Kramer, C. Yildiz, M. Nieger, S. Bräse, Eur. J. Org. Chem. 2014, 2014, 1287–1295.
- 42H. Song, Y. Li, Q-J. Yao, B.-F. Shi, Org. Chem. Front. 2022, 9, 4823–4828.
- 43J. Pu, L. Chen, R.-R. Wu, P. Ye, H.-L. Li, S. Wang, Z.-Y. Xu, S.-J. Lou, D.-Q. Xu, Chem. Commun. 2023, 59, 9348–9351.
- 44C. Pan, H. Sun, Y. Du, W. Wang, L. Xu, Y. Hu, M. Zhu, Org. Lett. 2024, 26, 2387–2392.
- 45J. Park, S. Chang, Chem.- Asian J. 2018, 13, 1089–1102.
- 46T. Yoshino, S. Matsunaga, Adv. Synth. Catal. 2017, 359, 1245–1262.
- 47A. de Meijere, B. König, Synlett 1997, 1997, 1221–1232.
- 48O. R. P. David, Tetrahedron 2012, 68, 8977–8993.
- 49A. de Meijere, B. Stulgies, K. Albrecht, K. Rauch, H. A. Wegner, H. Hopf, L. T. Scott, L. Eshdat, I. Aprahamian, M. Rabinovitz, Pure Appl. Chem. 2006, 78, 813–830.
- 50L. Chu, K.-J. Xiao, J.-Q. Yu, Science 2014, 346, 451–455.
- 51Q.-J. Yao, J.-H. Chen, H. Song, F.-R. Huang, B.-F. Shi, Angew. Chem. Int. Ed. 2022, 61, e202202892.
- 52J.-H. Chen, M.-Y. Teng, F.-R. Huang, H. Song, Z.-K. Wang, H.-L. Zhuang, Y.-J. Wu, X. Wu, Q.-J. Yao, B.-F. Shi, Angew. Chem. Int. Ed. 2022, 61, e202210106.
- 53Q.-J. Yao, F.-R. Huang, J.-H. Chen, M.-Y. Zhong, B. F. Shi, Angew. Chem. Int. Ed. 2023, 62, e202218533.
- 54G. Zhou, J.-H. Chen, Q.-J. Yao, F.-R. Huang, Z.-K. Wang, B.-F. Shi, Angew. Chem. Int. Ed. 2023, 62, e202302964.
- 55P.-F. Qian, G. Zhou, J.-H. Hu, B.-J. Wang, A.-L. Jiang, T. Zhou, W.-K. Yuan, Q.-J. Yao, J.-H. Chen, K.-X. Kong, B.-F. Shi, Angew. Chem. Int. Int. 2024, 63, e202412459.
- 56Y.-J. Wu, J.-H. Chen, M.-Y. Teng, X. Li, T.-Y. Jiang, F.-R. Huang, Q.-J. Yao, B.-F. Shi, J. Am. Chem. Soc. 2023, 145, 2449.
- 57F.-R. Huang, P. Zhang,Q.-J. Yao, B.-F. Shi, CCS Chem. 2024, 6, 2783–2793.
- 58F.-R. Huang, Q.-J. Yao, P. Zhang, M.-Y. Teng, J.-H. Chen, L.-C. Jiang, B.-F. Shi, J. Am. Chem. Soc. 2024, 146, 15576-15586.
- 59M.-Y. Teng, D.-Y. Liu, S.-Y. Mao, X. Wu, J.-H. Chen, M.-Y. Zhong, F.-R. Huang, Q.-J. Yao, B. F. Shi, Angew. Chem. Int. Ed. 2024, 63, e202407640.
- 60 For a personal account, see: Q.-J. Yao, B.-F. Shi, Acc. Chem. Res. 2025, 58, 971–990.
- 61X.-J. Si, D. Yang, M.-C. Sun, D. Wei, M.-P. Song, J.-L. Niu, Nat. Synth. 2022, 1, 709–718.
- 62T. Li, L. Shi, X. Wang, C. Yang, D. Yang, M.-P. Song, J.-L. Niu, Nat. Commun. 2023, 14, 5217.
- 63T. Liu, W. Zhang, C. Xu, Z. Xu, D. Song, W. Qian, G. Lu, C.-J. Zhang, W. Zhong, F. Ling, Green Chem. 2023, 25, 3606–3614.
- 64Y. Zhang, S.-L. Liu, T. Li, M. Xu, Q. Wang, D. Yang, M.-P. Song, J.-L. Niu, ACS Catal. 2024, 14, 1–9.
- 65A. Das, S. Kumaran, H. S. Ravi Sankar, J. R. Premkumar, B. Sundararaju, Angew. Chem. Int. Ed. 2024, 63, e202406195.
- 66G. Zaitsev, D. Shabashov, O. Daugulis, J. Am. Chem. Soc. 2005, 127, 13154–13155.
- 67O. Daugulis, J. Roane, L. D. Tran, Acc. Chem. Res. 2015, 48, 1053–1064.
- 68L. Lukasevics, G. N. Oh, X. Wang, L. Grigorjeva, O. Daugulis, J. Am. Chem. Soc. 2025, 147, 2476–2490.
- 69 Deposition numbers 2346769 [(Sp)-1a], 2346728 (3ab), 2415716 (3as), 2415717 (9), and 2430713 (Co-1-CPy) contain the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service.
- 70X. Zhang, G. Lu, M. Sun, M. Mahankali, Y. Ma, M. Zhang, W. Hua, Y. Hu, Q. Wang, J. Chen, G. He, X. Qi, W. Shen, P. Liu, G. Chen, Nat. Chem. 2018, 10, 540–548.
- 71M. M. Haque, D. P. Murale, Y. K. Kim, J.-S. Lee, Int. J. Mol. Sci. 2019, 20, 1959.
- 72M. Nakagawa, Y. Matsuki, K. Nagao, H. Ohmiya, J. Am. Chem. Soc. 2022, 144, 7953–7959.
- 73J. Liu, J. Rong, D. P. Wood, Y. Wang, S. H. Liang, S. Lin, J. Am. Chem. Soc. 2024, 146, 4380–4392.
- 74H.-L. Sun, F. Yang, W.-T. Ye, J.-J. Wang, R. Zhu, ACS Catal. 2020, 10, 4983–4989.