Crystalline Silagermenides as Powerful Synthons: Unraveling π-Bonding and Lone Pair Effects in the Multiple Bonds of Heavier Main Group Analogs of the Vinyl Anion
Xue-Yi He
Department of Chemistry and Research Center for Chemical Biology and Omics Analysis, College of Science, Southern University of Science and Technology, Shenzhen, 518055 China
Search for more papers by this authorQiuming Liang
Department of Chemistry and Dongguan Key Laboratory for Data Science and Intelligent Medicine, Great Bay University, Dongguan, 523000 China
Search for more papers by this authorCorresponding Author
Yanbo Mei
Department of Chemistry and Research Center for Chemical Biology and Omics Analysis, College of Science, Southern University of Science and Technology, Shenzhen, 518055 China
Department of Chemistry and Dongguan Key Laboratory for Data Science and Intelligent Medicine, Great Bay University, Dongguan, 523000 China
E-mail: [email protected]; [email protected]
Search for more papers by this authorCorresponding Author
Liu Leo Liu
Department of Chemistry and Research Center for Chemical Biology and Omics Analysis, College of Science, Southern University of Science and Technology, Shenzhen, 518055 China
E-mail: [email protected]; [email protected]
Search for more papers by this authorXue-Yi He
Department of Chemistry and Research Center for Chemical Biology and Omics Analysis, College of Science, Southern University of Science and Technology, Shenzhen, 518055 China
Search for more papers by this authorQiuming Liang
Department of Chemistry and Dongguan Key Laboratory for Data Science and Intelligent Medicine, Great Bay University, Dongguan, 523000 China
Search for more papers by this authorCorresponding Author
Yanbo Mei
Department of Chemistry and Research Center for Chemical Biology and Omics Analysis, College of Science, Southern University of Science and Technology, Shenzhen, 518055 China
Department of Chemistry and Dongguan Key Laboratory for Data Science and Intelligent Medicine, Great Bay University, Dongguan, 523000 China
E-mail: [email protected]; [email protected]
Search for more papers by this authorCorresponding Author
Liu Leo Liu
Department of Chemistry and Research Center for Chemical Biology and Omics Analysis, College of Science, Southern University of Science and Technology, Shenzhen, 518055 China
E-mail: [email protected]; [email protected]
Search for more papers by this authorGraphical Abstract
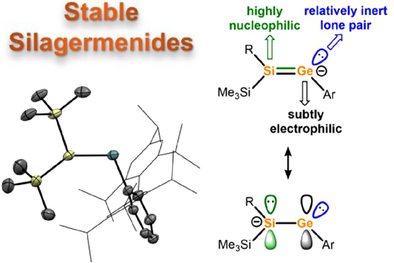
Acyclic silagermenides are synthesized through a straightforward desilylation process. Their electronic properties are explored using DFT calculations, revealing a high-lying and polarized Si─Ge π bond. Reactions with electrophiles lead to nucleophilic substitution at the Si atom, giving germylene intermediates, which then undergo further transformation into unique main group compounds and unprecedented metal complexes.
Abstract
Compared to common vinyl anions, their heavier heteronuclear analog, silagermenides [R2Si═GeR]ˉ, remain exceedingly rare. Herein, we present a systematic investigation of silagermenides, synthesized via a straightforward desilylation route. We delve into the bonding characteristics, revealing a weak, polarized Si─Ge π bond with a significant nonbonded lone pair character at the β-Si position. This β-Si exhibits predominantly nucleophilic behavior, while the α-Ge position demonstrates subtly electrophilic tendencies, despite the presence of a vinylic, formally anionic Ge atom. This leads to the formation of silagermenide complexes in an unprecedented η2 coordination mode, as well as various silagermenes and germylenes with unconventional substituents. We also document the facile cleavage of the ambiphilic Si═Ge double bond, resulting in the transfer of a formal doubly reduced silylene and a formal germyliumylidene. Our findings expand the understanding of heavier main group analogs of the vinyl anion, with important implications for their synthesis and reactivity.
Conflict of Interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the Supporting Information of this article.
Supporting Information
| Filename | Description |
|---|---|
| anie202505940-supp-0001-SuppMat.pdf9 MB | Supporting.Information |
| anie202505940-sup-0002-SuppMat.zip14.6 MB | Supporting.Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1W. E. Dasent, J. Chem. Educ. 1963, 40, 130.
- 2D. E. Goldberg, D. H. Harris, M. F. Lappert, K. M. Thomas, J. Chem. Soc. Chem. Commun. 1976, 261.
- 3R. West, M. J. Fink, J. Michl, Science 1981, 214, 1343–1344.
- 4M. Yoshifuji, I. Shima, N. Inamoto, K. Hirotsu, T. Higuchi, J. Am. Chem. Soc. 1981, 103, 4587–4589.
- 5A. G. Brook, F. Abdesaken, B. Gutekunst, G. Gutekunst, R. K. Kallury, J. Chem. Soc. Chem. Commun. 1981, 191–192.
- 6G. Becker, G. Gresser, W. Uhl, Z. Naturforsch B 1981, 36, 16–19.
- 7P. P. Power, Chem. Rev. 1999, 99, 3463–3504.
- 8R. C. Fischer, P. P. Power, Chem. Rev. 2010, 110, 3877–3923.
- 9V. Nesterov, D. Reiter, P. Bag, P. Frisch, R. Holzner, A. Porzelt, S. Inoue, Chem. Rev. 2018, 118, 9678–9842.
- 10L. Zhao, S. Pan, N. Holzmann, P. Schwerdtfeger, G. Frenking, Chem. Rev. 2019, 119, 8781–8845.
- 11P. P. Power, Organometallics 2020, 39, 4127–4138.
- 12C. Weetman, Chem. − Eur. J. 2021, 27, 1941–1954.
- 13K. Ota, R. Kinjo, Chem. Soc. Rev. 2021, 50, 10594–10673.
- 14R. S. Ghadwal, Angew. Chem. Int. Ed. 2023, 62, e202304665.
- 15P. P. Power, Nature 2010, 463, 171–177.
- 16C. Präsang, D. Scheschkewitz, Chem. Soc. Rev. 2016, 45, 900–921.
- 17A. M. Priegert, B. W. Rawe, S. C. Serin, D. P. Gates, Chem. Soc. Rev. 2016, 45, 922–953.
- 18C. Weetman, S. Inoue, ChemCatChem 2018, 10, 4213–4228.
- 19J.-D. Guo, T. Sasamori, Chem. Asian J. 2018, 13, 3800–3817.
- 20R. L. Melen, Science 2019, 363, 479–484.
- 21F. Ebeler, B. Neumann, H.-G. Stammler, I. Fernández, R. S. Ghadwal, J. Am. Chem. Soc. 2024, 146, 34979–34989.
- 22B. A. Feit, U. Melamed, R. R. Schmidt, H. Speer, J. Chem. Soc. Perkin Trans. 1 1981, 1329–1338.
- 23M. Braun, Angew. Chem. Int. Ed. 1998, 37, 430–451.
10.1002/(SICI)1521-3773(19980302)37:4<430::AID-ANIE430>3.0.CO;2-5 PubMed Web of Science® Google Scholar
- 24G. Boche, J. C. W. Lohrenz, Chem. Rev. 2001, 101, 697–756.
- 25R. Knorr, Chem. Rev. 2004, 104, 3795–3850.
- 26M. M. D. Roy, E. Rivard, Acc. Chem. Res. 2017, 50, 2017–2025.
- 27R. S. Ghadwal, Acc. Chem. Res. 2022, 55, 457–470.
- 28D. Bravo-Zhivotovskii, R. Dobrovetsky, D. Nemirovsky, V. Molev, M. Bendikov, G. Molev, M. Botoshansky, Y. Apeloig, Angew. Chem. Int. Ed. 2008, 47, 4343–4345.
- 29L. Zborovsky, R. Dobrovetsky, M. Botoshansky, D. Bravo-Zhivotovskii, Y. Apeloig, J. Am. Chem. Soc. 2012, 134, 18229–18232.
- 30D. Pinchuk, J. Mathew, A. Kaushansky, D. Bravo-Zhivotovskii, Y. Apeloig, Angew. Chem. Int. Ed. 2016, 55, 10258–10262.
- 31M. Ichinohe, K. Sanuki, S. Inoue, A. Sekiguchi, Organometallics 2004, 23, 3088–3090.
- 32S. Inoue, M. Ichinohe, A. Sekiguchi, Chem. Lett. 2005, 34, 1564–1565.
- 33M. Ichinohe, K. Sanuki, S. Inoue, A. Sekiguchi, Silicon Chem. 2007, 3, 111–116.
- 34H. Yasuda, V. Y. Lee, A. Sekiguchi, J. Am. Chem. Soc. 2009, 131, 6352–6353.
- 35D. Scheschkewitz, Angew. Chem. Int. Ed. 2004, 43, 2965–2967.
- 36K. Abersfelder, D. Güclü, D. Scheschkewitz, Angew. Chem. Int. Ed. 2006, 45, 1643–1645.
- 37P. Willmes, M. J. Cowley, M. Hartmann, M. Zimmer, V. Huch, D. Scheschkewitz, Angew. Chem. Int. Ed. 2014, 53, 2216–2220.
- 38P. Willmes, L. Junk, V. Huch, C. B. Yildiz, D. Scheschkewitz, Angew. Chem. Int. Ed. 2016, 55, 10913–10917.
- 39N. M. Obeid, L. Klemmer, D. Maus, M. Zimmer, J. Jeck, I. Bejan, A. J. P. White, V. Huch, G. Jung, D. Scheschkewitz, Dalton Trans. 2017, 46, 8839–8848.
- 40Y. Heider, P. Willmes, D. Mühlhausen, L. Klemmer, M. Zimmer, V. Huch, D. Scheschkewitz, Angew. Chem. Int. Ed. 2019, 58, 1939–1944.
- 41N. Parvin, Ankur, B. M., D. Scheschkewitz, Angew. Chem. Int. Ed. 2025, 64, e202422007.
- 42T. Iwamoto, M. Kobayashi, K. Uchiyama, S. Sasaki, S. Nagendran, H. Isobe, M. Kira, J. Am. Chem. Soc. 2009, 131, 3156–3157.
- 43T. Iwamoto, N. Akasaka, S. Ishida, Nat. Commun. 2014, 5, 5353.
- 44T. Kosai, S. Ishida, T. Iwamoto, J. Am. Chem. Soc. 2017, 139, 99–102.
- 45T. Kosai, T. Iwamoto, J. Am. Chem. Soc. 2017, 139, 18146–18149.
- 46T. Kosai, S. Ishida, T. Iwamoto, Dalton Trans. 2017, 46, 11271–11281.
- 47N. Akasaka, K. Fujieda, E. Garoni, K. Kamada, H. Matsui, M. Nakano, T. Iwamoto, Organometallics 2018, 37, 172–175.
- 48K. Tanaka, N. Akasaka, T. Kosai, S. Honda, Y. Ushijima, S. Ishida, T. Iwamoto, Molecules 2021, 26, 1632.
- 49D. Scheschkewitz, Chem. Lett. 2011, 40, 2–11.
- 50M. Tian, J. Zhang, H. Yang, C. Cui, J. Am. Chem. Soc. 2020, 142, 4131–4135.
- 51M. Tian, J. Zhang, L. Guo, C. Cui, Chem. Sci. 2021, 12, 14635–14640.
- 52P. Ghana, M. I. Arz, U. Das, G. Schnakenburg, A. C. Filippou, Angew. Chem. Int. Ed. 2015, 54, 9980–9985.
- 53T.-l. Nguyen, D. Scheschkewitz, J. Am. Chem. Soc. 2005, 127, 10174–10175.
- 54K. Abersfelder, T. l. Nguyen, D. Scheschkewitz, Z. Anorg. Allg. Chem. 2009, 635, 2093–2098.
- 55M. J. Cowley, K. Abersfelder, A. J. P. White, M. Majumdar, D. Scheschkewitz, Chem. Commun. 2012, 48, 6595–6597.
- 56J. Park, S. A. Batcheller, S. Masamune, J. Organomet. Chem. 1989, 367, 39–45.
- 57H. Schäfer, W. Saak, M. Weidenbruch, Angew. Chem. Int. Ed. 2000, 39, 3703–3705.
10.1002/1521-3773(20001016)39:20<3703::AID-ANIE3703>3.0.CO;2-9 CAS PubMed Web of Science® Google Scholar
- 58D. Nieder, L. Klemmer, Y. Kaiser, V. Huch, D. Scheschkewitz, Organometallics 2018, 37, 632–635.
- 59Y. Mizuhata, W. Ijichi, R. Nishino, T. Kato, E. Kayahara, S. Yamago, N. Tokitoh, Polyhedron 2023, 244, 116614.
- 60M. M. Siddiqui, S. Sinhababu, S. Dutta, S. Kundu, P. N. Ruth, A. Münch, R. Herbst-Irmer, D. Stalke, D. Koley, H. W. Roesky, Angew. Chem. Int. Ed. 2018, 57, 11776–11780.
- 61C. Gendy, J. Mikko Rautiainen, A. Mailman, H. M. Tuononen, Chem. − Eur. J. 2021, 27, 14405–14409.
- 62Y. Mizuhata, S. Fujimori, T. Sasamori, N. Tokitoh, Angew. Chem. Int. Ed. 2017, 56, 4588–4592.
- 63S. Fujimori, Y. Mizuhata, N. Tokitoh, Chem. − Eur. J. 2018, 24, 17039–17045.
- 64S. Fujimori, Y. Mizuhata, N. Tokitoh, Chem. Lett. 2018, 47, 708–710.
- 65S. Fujimori, Y. Mizuhata, N. Tokitoh, Chem. − Eur. J. 2019, 25, 6284–6289.
- 66Y. Goldshtein, Y. Glagovsky, B. Tumanskii, N. Fridman, D. Bravo-Zhivotovskii, Y. Apeloig, Angew. Chem. Int. Ed. 2022, 61, e202202452.
- 67S. Kumar, L. R. Maurer, G. Schnakenburg, U. Das, A. C. Filippou, Angew. Chem. Int. Ed. 2024, 63, e202400227.
- 68V. Y. Lee, M. Ichinohe, A. Sekiguchi, N. Takagi, S. Nagase, J. Am. Chem. Soc. 2000, 122, 9034–9035.
- 69V. Y. Lee, M. Ichinohe, A. Sekiguchi, J. Am. Chem. Soc. 2000, 122, 12604–12605.
- 70V. Y. Lee, M. Ichinohe, A. Sekiguchi, J. Organomet. Chem. 2001, 636, 41–48.
- 71M. Ichinohe, Y. Arai, A. Sekiguchi, N. Takagi, S. Nagase, Organometallics 2001, 20, 4141–4143.
- 72A. Sekiguchi, R. Izumi, S. Ihara, M. Ichinohe, V. Y. Lee, Angew. Chem. Int. Ed. 2002, 41, 1598–1600.
10.1002/1521-3773(20020503)41:9<1598::AID-ANIE1598>3.0.CO;2-8 CAS PubMed Web of Science® Google Scholar
- 73K. M. Baines, J. A. Cooke, Organometallics 1992, 11, 3487–3488.
- 74V. Y. Lee, H. Yasuda, A. Sekiguchi, J. Am. Chem. Soc. 2007, 129, 2436–2437.
- 75M. Igarashi, M. ıchinohe, A. Sekiguchi, Heteroat. Chem 2008, 19, 649–653.
- 76T. Iwamoto, J. Okita, N. Yoshida, M. Kira, Silicon 2011, 2, 209–216.
- 77C. Wilhelm, D. Raiser, H. Schubert, C. P. Sindlinger, L. Wesemann, Inorg. Chem. 2021, 60, 9268–9272.
- 78V. Y. Lee, K. Takanashi, T. Matsuno, M. Ichinohe, A. Sekiguchi, Appl. Organomet. Chem. 2010, 24, 834–836.
- 79P. K. Majhi, V. Huch, D. Scheschkewitz, Angew. Chem. Int. Ed. 2021, 60, 242–246.
- 80M. Asay, C. Jones, M. Driess, Chem. Rev. 2011, 111, 354–396.
- 81M. He, C. Hu, R. Wei, X.-F. Wang, L. L. Liu, Chem. Soc. Rev. 2024, 53, 3896–3951.
- 82K. Gour, M. K. Bisai, S. S. Sen, Eur. J. Inorg. Chem. 2022, 2022, e202200071.
- 83C. Marschner, Eur. J. Inorg. Chem. 1998, 1998, 221–226.
10.1002/(SICI)1099-0682(199802)1998:2<221::AID-EJIC221>3.0.CO;2-G Google Scholar
- 84L. Pu, M. M. Olmstead, P. P. Power, B. Schiemenz, Organometallics 1998, 17, 5602–5606.
- 85M. Usher, A. V. Protchenko, A. Rit, J. Campos, E. L. Kolychev, R. Tirfoin, S. Aldridge, Chem. − Eur. J. 2016, 22, 11685–11698.
- 86 Deposition numbers 2421666 (for 1), 2421667 (for [K(THF)+2ˉ]2), 2421658 (for [K(18-C-6)(OEt2)]+2ˉ), 2421661 (for K(18-C-6)]+3ˉ), 2421668 (for 4), 2421669 (for K+5ˉ), 2421662 (for [K(18-C-6)]+5ˉ), 2421655 (for 6), 2421664 (for 7), 2421663 (for 8 and 9), 2421654 (for 10), 2421660 (for 11), 2421659 (for 12), 2421656 (for 13), 2421657 (for 14), 2421665 (for 15), These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service.
- 87Y. Segawa, M. Yamashita, K. Nozaki, Science 2006, 314, 113–115.
- 88L. Weber, Eur. J. Inorg. Chem. 2017, 2017, 3461–3488.
- 89C. Duan, C. Cui, Chem. Soc. Rev. 2024, 53, 361–379.
- 90P. Pyykkö, M. Atsumi, Chem. − Eur. J. 2009, 15, 12770–12779.
- 91G. Knizia, J. Chem. Theory Comput. 2013, 9, 4834–4843.
- 92A. Savin, R. Nesper, S. Wengert, T. F. Fässler, Angew. Chem. Int. Ed. Engl. 1997, 36, 1808–1832.
- 93M. Kosa, M. Karni, Y. Apeloig, J. Am. Chem. Soc. 2013, 135, 9032–9040.
- 94G. Trinquier, J. P. Malrieu, P. Riviere, J. Am. Chem. Soc. 1982, 104, 4529–4533.
- 95E. A. Carter, W. A. Goddard, III, J. Phys. Chem. 1986, 90, 998–1001.
- 96G. Trinquier, J. P. Malrieu, J. Am. Chem. Soc. 1987, 109, 5303–5315.
- 97J. P. Malrieu, G. Trinquier, J. Am. Chem. Soc. 1989, 111, 5916–5921.
- 98T. Ziegler, A. Rauk, Theoret. Chim. Acta 1977, 46, 1–10.
- 99M. Mitoraj, A. Michalak, J. Mol. Model. 2008, 14, 681–687.
- 100Pauling, L., The Nature of the Chemical Bond: An Introduction to Modern Structural Chemistry, 3rd ed.; Cornell University Press, Ithaca, NY 1960.
- 101L. Komorowski, J. Lipinski, M. J. Pyka, J. Phys. Chem. 1993, 97, 3166–3170.
- 102F. De Proft, W. Langenaeker, P. Geerlings, J. Phys. Chem. 1993, 97, 1826–1831.
- 103D. Sorbelli, L. Belpassi, P. Belanzoni, J. Am. Chem. Soc. 2021, 143, 14433–14437.
- 104C. Morell, A. Grand, A. Toro-Labbé, J. Phys. Chem. A 2005, 109, 205–212.
- 105J. Li, Y. Mei, L. L. Liu, Eur. J. Inorg. Chem. 2022, 2022, e202200368.
- 106S. Stigler, S. Fujimori, A. Kostenko, S. Inoue, Chem. Sci. 2024, 15, 4275–4291.
- 107T. J. Hadlington, Chem. Soc. Rev. 2024, 53, 9718–9737.
- 108M. Wilfling, K. W. Klinkhammer, Angew. Chem. Int. Ed. 2010, 49, 3219–3223.
- 109L. Pu, B. Twamley, S. T. Haubrich, M. M. Olmstead, B. V. Mork, R. S. Simons, P. P. Power, J. Am. Chem. Soc. 2000, 122, 650–656.
- 110L. Pu, P. P. Power, I. Boltes, R. Herbst-Irmer, Organometallics 2000, 19, 352–356.
- 111B. E. Eichler, A. D. Phillips, S. T. Haubrich, B. V. Mork, P. P. Power, Organometallics 2002, 21, 5622–5627.
- 112P. G. Hayes, C. W. Gribble, R. Waterman, T. D. Tilley, J. Am. Chem. Soc. 2009, 131, 4606–4607.
- 113H.-J. Liu, J. Guihaumé, T. Davin, C. Raynaud, O. Eisenstein, T. D. Tilley, J. Am. Chem. Soc. 2014, 136, 13991–13994.
- 114M. M. Juckel, J. Hicks, D. Jiang, L. Zhao, G. Frenking, C. Jones, Chem. Commun. 2017, 53, 12692–12695.
- 115S. Takahashi, E. Bellan, A. Baceiredo, N. Saffon-Merceron, S. Massou, N. Nakata, D. Hashizume, V. Branchadell, T. Kato, Angew. Chem. Int. Ed. 2019, 58, 10310–10314.
- 116P. W. Smith, R. C. Handford, T. D. Tilley, Organometallics 2019, 38, 4060–4065.
- 117A. C. Phung, J. C. Fettinger, P. P. Power, Organometallics 2021, 40, 3472–3479.
- 118R. C. Handford, M. A. Nesbit, P. W. Smith, R. D. Britt, T. D. Tilley, J. Am. Chem. Soc. 2022, 144, 358–367.
- 119R. Wei, S. Ju, L. L. Liu, Angew. Chem. Int. Ed. 2022, 61, e202205618.
- 120C. Hu, X.-F. Wang, C. Hu, R. Wei, H. Wang, L. L. Liu, Acc. Chem. Res. 2025, 58, 452–462.
- 121J. Hicks, A. Mansikkamäki, P. Vasko, J. M. Goicoechea, S. Aldridge, Nat. Chem. 2019, 11, 237–241.
- 122A. Suzuki, X. Guo, Z. Lin, M. Yamashita, Chem. Sci. 2021, 12, 917–928.