Bromide as Noninnocent Ligand to Iron Tames Fenton Chemistry for Chemoselective Nondegrading Oxidation
Corresponding Author
Guodong Zhao
School of Chinese Materia Medica, Beijing University of Chinese Medicine, Beijing, 102488 China
Engineering Research Center for Pharmaceutics of Chinese Materia Medica and New Drug Development, Ministry of Education, Beijing, China
These authors contributed equally to this work.
E-mail: [email protected]; [email protected]; [email protected]
Search for more papers by this authorKang Xue
School of Chinese Materia Medica, Beijing University of Chinese Medicine, Beijing, 102488 China
These authors contributed equally to this work.
Search for more papers by this authorHuiling Dong
School of Chinese Materia Medica, Beijing University of Chinese Medicine, Beijing, 102488 China
These authors contributed equally to this work.
Search for more papers by this authorShaoyan Lou
School of Chinese Materia Medica, Beijing University of Chinese Medicine, Beijing, 102488 China
Search for more papers by this authorXiaohui Zhang
School of Chinese Materia Medica, Beijing University of Chinese Medicine, Beijing, 102488 China
Search for more papers by this authorZhuo Cao
School of Chinese Materia Medica, Beijing University of Chinese Medicine, Beijing, 102488 China
Search for more papers by this authorCorresponding Author
Bingqing Yi
School of Chinese Materia Medica, Beijing University of Chinese Medicine, Beijing, 102488 China
E-mail: [email protected]; [email protected]; [email protected]
Search for more papers by this authorCorresponding Author
Rongbiao Tong
Department of Chemistry, The Hong Kong University of Science and Technology, Clearwater Bay, Kowloon, Hong Kong, China
E-mail: [email protected]; [email protected]; [email protected]
Search for more papers by this authorCorresponding Author
Guodong Zhao
School of Chinese Materia Medica, Beijing University of Chinese Medicine, Beijing, 102488 China
Engineering Research Center for Pharmaceutics of Chinese Materia Medica and New Drug Development, Ministry of Education, Beijing, China
These authors contributed equally to this work.
E-mail: [email protected]; [email protected]; [email protected]
Search for more papers by this authorKang Xue
School of Chinese Materia Medica, Beijing University of Chinese Medicine, Beijing, 102488 China
These authors contributed equally to this work.
Search for more papers by this authorHuiling Dong
School of Chinese Materia Medica, Beijing University of Chinese Medicine, Beijing, 102488 China
These authors contributed equally to this work.
Search for more papers by this authorShaoyan Lou
School of Chinese Materia Medica, Beijing University of Chinese Medicine, Beijing, 102488 China
Search for more papers by this authorXiaohui Zhang
School of Chinese Materia Medica, Beijing University of Chinese Medicine, Beijing, 102488 China
Search for more papers by this authorZhuo Cao
School of Chinese Materia Medica, Beijing University of Chinese Medicine, Beijing, 102488 China
Search for more papers by this authorCorresponding Author
Bingqing Yi
School of Chinese Materia Medica, Beijing University of Chinese Medicine, Beijing, 102488 China
E-mail: [email protected]; [email protected]; [email protected]
Search for more papers by this authorCorresponding Author
Rongbiao Tong
Department of Chemistry, The Hong Kong University of Science and Technology, Clearwater Bay, Kowloon, Hong Kong, China
E-mail: [email protected]; [email protected]; [email protected]
Search for more papers by this authorGraphical Abstract
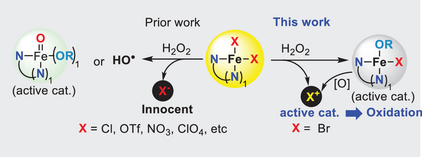
We report for the first time that the bromide ion, acting as a non-N-ligand in iron complexes with the tridentate N-ligand bis(2-pyridylmethyl)amine (BMPA), which is traditionally used in Fenton chemistry to degrade organic compounds, tames Fenton chemistry and enables chemo-selective, nondegrading oxidation reactions. This discovery of the bromide effect bridges the gap between Fenton chemistry and haloperoxidase-catalyzed halogenation, potentially inspiring further investigation into the “innocent” ligands of iron complexes for new reactivity and applications. The overlooked inorganic anion may be exploited in other catalytic systems.
Abstract
It has long been the chemistry dogma that the nitrogen-based ligand of iron complexes determines the redox reactivity; tetra- and/or pentadentate nitrogen-based ligand (N-ligand: PDP, porphyrin, N4Py) enables chemo-selective oxidation through high-valent iron species (FeIV/V═O), while bi- and/or tridentate N-ligand leads to the generation of highly reactive oxygen species (ROS) (i.e., hydroxyl radical) via a Fenton chemistry pathway. The effect of inorganic anionic ligands (i.e., halides, pseudohalides, triflate, nitrate, sulfate, etc) of these iron complexes has rarely been examined and overlooked as an “innocent” anion. Herein, we report our discovery that bromide (Br−) is not an innocent ligand to the iron-BPMA complexes [BMPA: bis(2-pyridylmethyl)amine] but a decisive factor for taming the Fenton chemistry (ROS) into a mild [HOBr] oxidant, which allows for chemo- and regioselective oxidation of furans, indoles, and sulfides without noticeable degradation. In contrast to the conventional Fenton chemistry pathway by many tridentate N-ligand iron complexes, our [Fe(BMPA)Br3] mimics haloperoxidases to generate HOBr by oxidation of bromide ion with hydrogen peroxide. The discovery of the bromide effect on iron complexes bridges the gap between Fenton chemistry and haloperoxidase-catalyzed halogenation and might stimulate interest in reinvestigating the “innocent” ligand of iron complexes for discovery of new reactivity and new applications. Additionally, the new catalytic system represents a mild and green oxidation method that might be useful in academia and industry.
Conflict of Interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.
Supporting Information
| Filename | Description |
|---|---|
| anie202505907-sup-0001-SuppMat.pdf17 MB | Supporting information |
| anie202505907-sup-0002-SuppMat.cif456.2 KB | Supporting information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1W. Nam, Acc. Chem. Res. 2007, 40, 522–531.
- 2J. Cho, S. Jeon, S. A. Wilson, L. V. Liu, E. A. Kang, J. J. Braymer, M. H. Lim, B. Hedman, K. O. Hodgson, J. S. Valentine, E. I. Solomon, W. Nam, Nature 2011, 478, 502–505.
- 3X. Lu, X.-X. Li, Y.-M. Lee, Y. Jang, M. S. Seo, S. Hong, K.-B. Cho, S. Fukuzumi, W. Nam, J. Am. Chem. Soc. 2020, 142, 3891–3904.
- 4S. Jana, P. De, C. Dey, S. G. Dey, A. Dey, S. S. Gupta, Chem. Sci. 2023, 14, 10515–10523.
- 5E. B. Bauer, Isr. J. Chem. 2017, 57, 1131–1150.
- 6Y. Zhang, M. Zhou, J. Hazard. Mater. 2019, 362, 436–450.
- 7U. J. Ahile, R. A. Wuana, A. U. Itodo, R. Sha'Ato, R. F. Dantas, Sci. Total Environ. 2020, 710, 134872.
- 8A. Checa-Fernandez, A. Santos, A. Romero, C. M. Dominguez, Catalysts 2021, 11, 722.
- 9S. Krishnan, C. A. Martínez-Huitle, P. V. Nidheesh, J. Environ. Chem. Eng. 2022, 10, 107183.
- 10H. Dong, W. Du, J. Dong, R. Che, F. Kong, W. Cheng, M. Ma, N. Gu, Y. Zhang, Nat. Commun. 2022, 13, 5365.
- 11J. Hohenberger, K. Ray, K. Meyer, Nat. Commun. 2012, 3, 720.
- 12W. N. Oloo, K. K. Meier, Y. Wang, S. Shaik, E. Münck, L. Que, Nat. Commun. 2014, 5, 3046.
- 13W. N. Oloo, L. Que, Acc. Chem. Res. 2015, 48, 2612–2621.
- 14L. Shu, J. C. Nesheim, K. Kauffmann, E. Münck, J. D. Lipscomb, L. Que, Science 1997, 275, 515–518.
- 15T. Jian, Y. Zhou, P. Wang, W. Yang, P. Mu, X. Zhang, X. Zhang, C.-L. Chen, Nat. Commun. 2022, 13, 3025.
- 16M. Borrell, E. Andris, R. Navrátil, J. Roithová, M. Costas, Nat. Commun. 2019, 10, 901.
- 17J. Chen, A. Draksharapu, D. Angelone, D. Unjaroen, S. K. Padamati, R. Hage, M. Swart, C. Duboc, W. R. Browne, ACS Catal. 2018, 8, 9665–9674.
- 18M. S. Chen, M. C. White, Science 2010, 327, 566–571.
- 19M. S. Chen, M. C. White, Science 2007, 318, 783–787.
- 20T. G. Traylor, F. Xu, J. Am. Chem. Soc. 1990, 112, 178–186.
- 21M. Borrell, M. Costas, J. Am. Chem. Soc. 2017, 139, 12821–12829.
- 22W. Zhu, A. Kumar, J. Xiong, M. J. Abernathy, X.-X. Li, M. S. Seo, Y.-M. Lee, R. Sarangi, Y. Guo, W. Nam, J. Am. Chem. Soc. 2023, 145, 4389–4393.
- 23J. Wei, L. Wu, H.-X. Wang, X. Zhang, C.-W. Tse, C.-Y. Zhou, J.-S. Huang, C.-M. Che, Angew. Chem. Int. Ed.2020, 59, 16561–16571.
- 24G. Olivo, O. Cussó, M. Costas, Chem-Asian J 2016, 11, 3148–3158.
- 25A. C. Lindhorst, S. Haslinger, F. E. Kühn, Chem. Commun. 2015, 51, 17193–17212.
- 26M. J. F. Calvete, M. Piñeiro, L. D. Dias, M. M. Pereira, ChemCatChem 2018, 10, 3615–3635.
- 27A. Kejriwal, J. Coord. Chem. 2022, 75, 937–971.
- 28G. Olivo, O. Cussó, M. Borrell, M. Costas, J. Biol. Inorg. Chem. 2017, 22, 425–452.
- 29O. Cussó, X. Ribas, M. Costas, Chem. Commun. 2015, 51, 14285–14298.
- 30Y.-X. Ye, C. Wen, J.-W. Wang, J. Pan, S. Huang, S. Liang, M. Zhou, Q. Tong, F. Zhu, J. Xu, G. Ouyang, Chem. Commun. 2020, 56, 5476–5479.
- 31S. S. F. Carvalho, N. M. F. Carvalho, Inorg. Chem. Commun. 2019, 108, 107507.
- 32S. A. Messele, C. Bengoa, F. E. Stüber, J. Giralt, A. Fortuny, A. Fabregat, J. Font, Catalysts 2019, 9, 474.
- 33Y. Hu, Y. Li, J. He, T. Liu, K. Zhang, X. Huang, L. Kong, J. Liu, J. Environ. Manage. 2018, 226, 256–263.
- 34I. Lampre, J.-L. Marignier, M. Mirdamadi-Esfahani, P. Pernot, P. Archirel, M. Mostafavi, J. Phys. Chem. A 2013, 117, 877–887.
- 35G. Zhao, Y. Wang, C. Wang, H. Lei, B. Yi, R. Tong, Green Chem. 2022, 24, 4041–4049.
- 36G. Zhao, E. Wang, R. Tong, ACS Sustainable Chem. Eng. 2021, 9, 6118–6125.
- 37G. Zhao, L. Liang, E. Wang, R. Tong, ACS Catal. 2021, 11, 3740–3748.
- 38G. Zhao, L. Liang, E. Wang, S. Lou, R. Qi, R. Tong, Green Chem. 2021, 23, 2300–2307.
- 39N. Lehnert, E. Kim, H. T. Dong, J. B. Harland, A. P. Hunt, E. C. Manickas, K. M. Oakley, J. Pham, G. C. Reed, V. S. Alfaro, Chem. Rev. 2021, 121, 14682–14905.
- 40H.-J. Krüger, Coordin. Chem. Rev. 2009, 253, 2450–2459.
- 41M. H. Lim, J.-U. Rohde, A. Stubna, M. R. Bukowski, M. Costas, R. Y. N. Ho, E. Münck, W. Nam, L. Que, Proc. Natl. Acad. Sci. USA 2003, 100, 3665–3670.
- 42N. M. F. Carvalho, A. Horn, O. A. C. Antunes, Appl. Catal. A-Gen. 2006, 305, 140–145.
- 43N. M. F. Carvalho, H. M. Alvarez, A. Horn, O. A. C. Antunes, Catal. Today 2008, 133, 689–694.
- 44G. C. Silva, N. M. F. Carvalho, A. Horn, E. R. Lachter, O. A. C. Antunes, J. Mol. Catal. A-Chem. 2017, 426, 564–571.
- 45 These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service.
- 46J. Xiao, S. Guo, D. Wang, Q. An, Chem. - Eur. J. 2024, 30, e202304337.
- 47H. L. Wiegand, C. T. Orths, K. Kerpen, H. V. Lutze, T. C. Schmidt, Environ. Sci. Technol. 2017, 51, 14321–14329.
- 48M. L. Kremer, Phys. Chem. Chem. Phys. 1999, 1, 3595–3605.
- 49D. H. R. Barton, D. Doller, Acc. Chem. Res. 1992, 25, 504–512.
- 50L. Liang, L.-D. Guo, R. Tong, Acc. Chem. Res. 2022, 55, 2326–2340.
- 51J. Yan, J.-L. Pozzo, A. Hamze, O. Provot, Eur. J. Org. Chem. 2022, 2022, e202101460.
- 52T. L. Gilchrist, C. J. Moody, Chem. Rev. 1977, 77, 409–435.
- 53R. Vanacore, L. Ham Amy-Joan, M. Voehler, R. Sanders Charles, P. Conrads Thomas, D. Veenstra Timothy, K. B. Sharpless, E. Dawson Philip, G. Hudson Billy, Science 2009, 325, 1230–1234.
- 54A. S. McCall, C. F. Cummings, G. Bhave, R. Vanacore, A. Page-McCaw, B. G. Hudson, Cell 2014, 157, 1380–1392.
- 55G. Bhave, C. F. Cummings, R. M. Vanacore, C. Kumagai-Cresse, I. A. Ero-Tolliver, M. Rafi, J.-S. Kang, V. Pedchenko, L. I. Fessler, J. H. Fessler, B. G. Hudson, Nat. Chem. Biol. 2012, 8, 784–790.
- 56T. Meng, L. A. Wells, T. Wang, J. Wang, S. Zhang, J. Wang, M. C. Kozlowski, T. Jia, J. Am. Chem. Soc. 2022, 144, 12476–12487.
- 57S. Zhou, T. Yan, Y. Li, Z. Jia, B. Wang, Y. Zhao, Y. Qiao, L. Xiong, Y. Li, Z. Li, Org. Biomol. Chem. 2014, 12, 6643–6652.
- 58O.-Y. Kang, E. Kim, W. H. Lee, D. H. Ryu, H. J. Lim, S. J. Park, RSC Adv. 2023, 13, 2004–2009.
- 59K. Xu, D. Luan, X. Wang, B. Hu, X. Liu, F. Kong, B. Tang, Angew. Chem. Int. Ed.2016, 55, 12751–12754.
- 60X. Tian, L. Song, M. Rudolph, F. Rominger, T. Oeser, A. S. K. Hashmi, Angew. Chem. Int. Ed.2019, 58, 3589–3593.
- 61J. Liu, X. Jia, L. Huang, Org. Lett. 2022, 24, 6772–6776.
- 62Q. Sun, C. Hüßler, J. Kahle, A. V. Mackenroth, M. Rudolph, P. Krämer, T. Oeser, A. S. K. Hashmi, Angew. Chem. Int. Ed.2024, 63, e202313738.
- 63K. O. Marichev, K. Wang, K. Dong, N. Greco, L. A. Massey, Y. Deng, H. Arman, M. P. Doyle, Angew. Chem. Int. Ed.2019, 58, 16188–16192.
- 64Q. Wang, S. Nara, A. Padwa, Org. Lett. 2005, 7, 839–841.
- 65F.-L. Haut, N. J. Feichtinger, I. Plangger, L. A. Wein, M. Müller, T.-N. Streit, K. Wurst, M. Podewitz, T. Magauer, J. Am. Chem. Soc. 2021, 143, 9002–9008.
- 66X. Xie, J. Sun, Org. Lett. 2021, 23, 8921–8925.
- 67Q. Cheng, Z. Bai, S. Tewari, T. Ritter, Nat. Chem. 2022, 14, 898–904.
- 68X. Tian, L. Song, A. S. K. Hashmi, Angew. Chem. Int. Ed.2020, 59, 12342–12346.
- 69S. Yoshida, T. Yano, Y. Misawa, Y. Sugimura, K. Igawa, S. Shimizu, K. Tomooka, T. Hosoya, J. Am. Chem. Soc. 2015, 137, 14071–14074.
- 70V. Bizet, R. Kowalczyk, C. Bolm, Chem. Soc. Rev. 2014, 43, 2426–2438.
- 71M. T. Passia, N. Bormann, J. S. Ward, K. Rissanen, C. Bolm, Angew. Chem. Int. Ed.2023, 62, e202305703.
- 72M. A. Warpehoski, V. S. Bradford, Tetrahedron Lett. 1986, 27, 2735–2738.
- 73H. Shimizu, K. Ikedo, K. Hamada, M. Ozawa, H. Matsumoto, K. Kamata, H. Nakamura, M. Ji, T. Kataoka, M. Hori, J. Chem. Soc., Perkin Trans. 1 1991, 1733–1747.
- 74H. Okamura, C. Bolm, Org. Lett. 2004, 6, 1305–1307.
- 75J. Gries, J. Krüger, Synlett 2014, 25, 1831–1834.
- 76M. Klein, S. R. Waldvogel, Angew. Chem. Int. Ed. 2021, 60, 23197–23201.
- 77M. Klein, D. L. Troglauer, S. R. Waldvogel, JACS Au 2023, 3, 575–583.
- 78S. Vivekananda, J. K. Wolken, F. Tureček, J. Phys. Chem. A 2001, 105, 9130–9141.
- 79M. S. Sharifi, H. Douroudgari, M. Vahedpour, Sci. Rep. 2021, 11, 13049.
- 80A. J. Carmichael, A. Samuni, P. Riesz, Photochem. Photobiol. 1985, 41, 635–642.
- 81Y. Guo, C. Zhou, L. Fang, Z. Liu, W. Li, M. Yang, ACS Omega 2021, 6, 8119–8130.
- 82K. Makino, T. Hagiwara, A. Murakami, Int. J. Radiat. Appl. Instrum., Part C 1991, 37, 657–665.
- 83J. R. Harbour, M. L. Hair, J. Phys. Chem. 1978, 82, 1397–1399.
- 84M. Kamranifar, S. Ghanbari, A. Fatehizadeh, E. Taheri, N. Azizollahi, Z. Momeni, M. Khiadani, K. Ebrahimpour, S. V. Ganachari, T. M. Aminabhavi, Environ. Pollut. 2024, 354, 124136.