Modulation of Photoluminescence of BODIHY Dye Using Water-Soluble Coordination Cages With Different Shapes
Valiyakath Abdul Rinshad
Department of Inorganic and Physical Chemistry, Indian Institute of Science, Bengaluru, 560012 India
Search for more papers by this authorPrajoy Kumar Mitra
School of Chemistry, Indian Institute of Science Education and Research, Thiruvananthapuram, 695551 India
Search for more papers by this authorSailendra Pradhan
Department of Inorganic and Physical Chemistry, Indian Institute of Science, Bengaluru, 560012 India
Search for more papers by this authorCorresponding Author
Dr. Yapamanu Adithya Lakshmanna
School of Chemistry, Indian Institute of Science Education and Research, Thiruvananthapuram, 695551 India
E-mail: [email protected]; [email protected]
Search for more papers by this authorCorresponding Author
Prof. Dr. Partha Sarathi Mukherjee
Department of Inorganic and Physical Chemistry, Indian Institute of Science, Bengaluru, 560012 India
E-mail: [email protected]; [email protected]
Search for more papers by this authorValiyakath Abdul Rinshad
Department of Inorganic and Physical Chemistry, Indian Institute of Science, Bengaluru, 560012 India
Search for more papers by this authorPrajoy Kumar Mitra
School of Chemistry, Indian Institute of Science Education and Research, Thiruvananthapuram, 695551 India
Search for more papers by this authorSailendra Pradhan
Department of Inorganic and Physical Chemistry, Indian Institute of Science, Bengaluru, 560012 India
Search for more papers by this authorCorresponding Author
Dr. Yapamanu Adithya Lakshmanna
School of Chemistry, Indian Institute of Science Education and Research, Thiruvananthapuram, 695551 India
E-mail: [email protected]; [email protected]
Search for more papers by this authorCorresponding Author
Prof. Dr. Partha Sarathi Mukherjee
Department of Inorganic and Physical Chemistry, Indian Institute of Science, Bengaluru, 560012 India
E-mail: [email protected]; [email protected]
Search for more papers by this authorGraphical Abstract
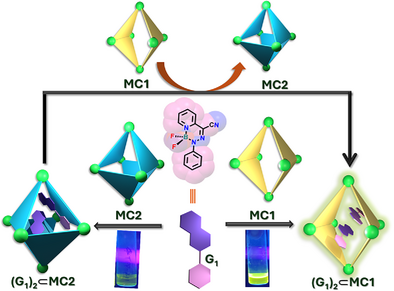
The fluorescence of Boron difluoride hydrazone (BODIHY) (G1) dye was effectively controlled through confinement within coordination cages of different shapes. The encapsulation of G1 within a double-square cage (MC1) resulted in a highly emissive solution, whereas similar confinement within an octahedral cage (MC2) exhibited a weak emission. Moreover, we achieved turn-on emission of G1 through the displacement of G1 from MC2 to MC1.
Abstract
The confinement of guest molecules within supramolecular hosts can alter their photophysical properties. However, the shapes of the hosts in regulating the guest's emission remains underexplored. Herein, we investigate how the shape of the host alters the emission behavior of boron difluoride hydrazone (BODIHY) (G1) dye encapsulated within two iso-stoichiometric water-soluble coordination cages: MC1 (double-square cage) and MC2 (octahedral cage). Encapsulation of G1 within MC1 results in a highly emissive solution, whereas similar confinement in MC2 leads to a non-emissive host–guest solution. A similar trend was observed with different sets of iso-stoichiometric cages MC3 (double-square cage) and MC4 (octahedral cage). Using a combination of femtosecond transient absorption and time-resolved fluorescence spectroscopy, we observed that the disparity in fluorescence behavior of BODIHY is attributed to charge transfer interactions between the guest and ligand panels of cages. The shape of the coordination cage dictates the preorganization of the guest within the cavity, thereby suppressing or promoting this charge transfer interactions. Moreover, we demonstrate a turn-on emission of BODIHY dye due to its preferential binding to a double-square cage. These findings provide fundamental insights into host-mediated modulation of the guest's photophysics and offer a blueprint for designing supramolecular systems with tunable emissive behavior.
Conflict of Interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
Data involved in this work are included in this article and the corresponding supplementary materials. The crystal structure of (G1)2⊂MC1 is available in the CCDC database under CCDC-2403903.
Supporting Information
| Filename | Description |
|---|---|
| anie202505772-sup-0001-SuppMatS1.pdf8.9 MB | The authors have cited additional references within the supporting information.[85-93] Supporting Information S1 |
| anie202505772-sup-0002-SuppMatS2.cif9.1 MB | Supporting Information S2 |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1L. Shang, F. Ye, M. Li, Y. Zhao, Chem. Soc. Rev. 2022, 51, 4075–4093.
- 2M. Zimmer, Chem. Rev. 2002, 102, 759–782.
- 3O. Shimomura, Angew. Chem. Int. Ed. 2009, 48, 5590–5602.
- 4Y. Wang, H. Wu, W. Hu, J. F. Stoddart, Adv. Mater. 2022, 34, 2105405.
- 5J. Rühe, K. Vinod, H. Hoh, K. Shoyama, M. Hariharan, F. Würthner, J. Am. Chem. Soc. 2024, 146, 28222–28232.
- 6G. A. Leith, C. R. Martin, J. M. Mayers, P. Kittikhunnatham, R. W. Larsen, N. B. Shustova, Chem. Soc. Rev. 2021, 50, 4382–4410.
- 7A. B. Grommet, M. Feller, R. Klajn, Nat. Nanotechnol. 2020, 15, 256–271.
- 8M. Gutiérrez, Y. Zhang, J.-C. Tan, Chem. Rev. 2022, 122, 10438–10483.
- 9J. Li, S. Yuan, J.-S. Qin, L. Huang, R. Bose, J. Pang, P. Zhang, Z. Xiao, K. Tan, A. V. Malko, T. Cagin, H.-C. Zhou, ACS Appl. Mater. Interfaces 2020, 12, 26727–26732.
- 10R. Chakrabarty, P. S. Mukherjee, P. J. Stang, Chem. Rev. 2011, 111, 6810–6918.
- 11T. R. Cook, P. J. Stang, Chem. Rev. 2015, 115, 7001–7045.
- 12W. Zhou, T. Sarma, Y. Su, C. Lei, J. L. Sessler, Chem. Sci. 2022, 13, 692–697.
- 13H. Zhang, J. Lee, A. D. Lammer, X. Chi, J. T. Brewster, V. M. Lynch, H. Li, Z. Zhang, J. L. Sessler, J. Am. Chem. Soc. 2016, 138, 4573–4579.
- 14H. Liu, C. Guo, L. Li, Z. Zhang, Y. Hou, C. Mu, G.-l. Hou, Z. Zhang, H. Wang, X. Li, M. Zhang, J. Am. Chem. Soc. 2024, 146, 15787–15795.
- 15M. Zhang, M. L. Saha, M. Wang, Z. Zhou, B. Song, C. Lu, X. Yan, X. Li, F. Huang, S. Yin, P. J. Stang, J. Am. Chem. Soc. 2017, 139, 5067–5074.
- 16L.-J. Chen, H.-B. Yang, Acc. Chem. Res. 2018, 51, 2699–2710.
- 17E. G. Percástegui, T. K. Ronson, J. R. Nitschke, Chem. Rev. 2020, 120, 13480–13544.
- 18M. Yamashina, Y. Sei, M. Akita, M. Yoshizawa, Nat. Commun 2014, 5, 4662.
- 19V. J. Pastore, M. G. Sullivan, H. K. Welgama, M. R. Crawley, A. E. Friedman, C. Rumsey, M. Trebbin, J. Rzayev, T. R. Cook, Chem. Mater. 2023, 35, 1651–1658.
- 20M. Han, D. M. Engelhard, G. H. Clever, Chem. Soc. Rev. 2014, 43, 1848–1860.
- 21L. Ma, C. J. Haynes, A. B. Grommet, A. Walczak, C. C. Parkins, C. M. Doherty, L. Longley, A. Tron, A. R. Stefankiewicz, T. D. Bennett, J. R. Nitschke, Nat. Chem. 2020, 12, 270–275.
- 22W.-X. Gao, H.-J. Feng, B.-B. Guo, Y. Lu, G.-X. Jin, Chem. Rev. 2020, 120, 6288–6325.
- 23J. Zhou, L. Rao, G. Yu, T. R. Cook, X. Chen, F. Huang, Chem. Soc. Rev. 2021, 50, 2839–2891.
- 24V. Martí-Centelles, A. L. Lawrence, P. J. Lusby, J. Am. Chem. Soc. 2018, 140, 2862–2868.
- 25F. Yin, J. Yang, L.-P. Zhou, X. Meng, C.-B. Tian, Q.-F. Sun, J. Am. Chem. Soc. 2024, 146, 7811–7821.
- 26D. Xu, Y. Li, S. Yin, F. Huang, Chem. Soc. Rev. 2024, 53, 3167–3204.
- 27R.-J. Li, A. Tarzia, V. Posligua, K. E. Jelfs, N. Sanchez, A. Marcus, A. Baksi, G. H. Clever, F. Fadaei-Tirani, K. Severin, Chem. Sci. 2022, 13, 11912–11917.
- 28Y. Li, J. He, G. Lu, C. Wang, M. Fu, J. Deng, F. Yang, D. Jiang, X. Chen, Z. Yu, Y. Liu, C. Yu, Y. Cui, Nat. Commun 2024, 15, 7044.
- 29Y. Xu, C. Li, X. Ma, W. Tuo, L. Tu, X. Li, Y. Sun, P. J. Stang, Y. Sun, Proc. Natl. Acad. Sci. U.S.A. 2022, 119, e2209904119.
- 30N. Kishi, M. Akita, M. Yoshizawa, Angew. Chem. Int. Ed. 2014, 53, 3604–3607.
- 31M. Han, R. Michel, B. He, Y. S. Chen, D. Stalke, M. John, G. H. Clever, Angew. Chem. Int. Ed. 2013, 52, 1319–1323.
- 32H. Takezawa, M. Fujita, Bull. Chem. Soc. Jpn. 2021, 94, 2351–2369.
- 33J. S. Train, A. B. Wragg, A. J. Auty, A. J. Metherell, D. Chekulaev, C. G. Taylor, S. P. Argent, J. A. Weinstein, M. D. Ward, Inorg. Chem. 2019, 58, 2386–2396.
- 34O. Yanshyna, M. J. Białek, O. V. Chashchikhin, R. Klajn, Commun. Chem. 2022, 5, 44.
- 35L.-J. Wang, S. Bai, Y.-F. Han, J. Am. Chem. Soc. 2022, 144, 21244–21254.
- 36E. A. Dolgopolova, A. A. Berseneva, M. S. Faillace, O. A. Ejegbavwo, G. A. Leith, S. W. Choi, H. N. Gregory, A. M. Rice, M. D. Smith, M. Chruszcz, S. Garashchuck, K. Mythreye, N. B. Shustova, J. Am. Chem. Soc. 2020, 142, 4769–4783.
- 37A. N. Bismillah, I. Aprahamian, J. Phys. Org. Chem. 2023, 36, e4485.
- 38D. Cappello, A. E. Watson, J. B. Gilroy, Macromol. Rapid Commun. 2021, 42, 2000553.
- 39D. Cappello, F. L. Buguis, P. D. Boyle, J. B. Gilroy, ChemPhotoChem 2022, 6, e202200131.
- 40D. Cappello, F. L. Buguis, J. B. Gilroy, ACS Omega 2022, 7, 32727–32739.
- 41X. Su, I. Aprahamian, Chem. Soc. Rev. 2014, 43, 1963.
- 42C. Yu, X. Fang, Q. Wu, L. Jiao, L. Sun, Z. Li, P.-K. So, W.-Y. Wong, E. Hao, Org. Lett. 2020, 22, 4588–4592.
- 43Y. Chen, J. W. Lam, R. T. Kwok, B. Liu, B. Z. Tang, Mater. Horiz. 2019, 6, 428–433.
- 44Y. Hong, J. W. Lam, B. Z. Tang, Chem. Soc. Rev. 2011, 40, 5361.
- 45M. A. Haidekker, E. A. Theodorakis, Org. Biomol. Chem. 2007, 5, 1669–1678.
- 46H. Qian, M. E. Cousins, E. H. Horak, A. Wakefield, M. D. Liptak, I. Aprahamian, Nat. Chem. 2017, 9, 83–87.
- 47M. Fujita, D. Oguro, M. Miyazawa, H. Oka, K. Yamaguchi, K. Ogura, Nature 1995, 378, 469–471.
- 48D. Samanta, S. Mukherjee, Y. P. Patil, P. S. Mukherjee, Chem. - Eur. J. 2012, 18, 12322–12329.
- 49M. Fujita, S. Y. Yu, T. Kusukawa, H. Funaki, K. Ogura, K. Yamaguchi, Angew. Chem. Int. Ed. 1998, 37, 2082–2085.
10.1002/(SICI)1521-3773(19980817)37:15<2082::AID-ANIE2082>3.0.CO;2-0 CAS PubMed Web of Science® Google Scholar
- 50D. Chakraborty, S. Ali, P. Choudhury, N. Hickey, P. S. Mukherjee, J. Am. Chem. Soc. 2023, 145, 26973–26982.
- 51Y. Yang, X. Su, C. N. Carroll, I. Aprahamian, Chem. Sci. 2012, 3, 610–613.
- 52J. Wang, L. Avram, Y. Diskin-Posner, M. J. Białek, W. Stawski, M. Feller, R. Klajn, J. Am. Chem. Soc. 2022, 144, 21244–21254.
- 53J. Gemen, J. R. Church, T.-P. Ruoko, N. Durandin, M. J. Białek, M. Weißenfels, M. Feller, M. Kazes, M. Odaybat, V. A. Borin, R. Kalepu, Y. D. Posner, D. Oron, M. J. Fuchter, A. Priimagi, I. Schapiro, R. Klajn, Science 2023, 381, 1357–1363.
- 54J. Gemen, M. J. Białek, M. Kazes, L. J. Shimon, M. Feller, S. N. Semenov, Y. Diskin-Posner, D. Oron, R. Klajn, Chem 2022, 8, 2362–2379.
- 55J. Gemen, J. Ahrens, L. J. Shimon, R. Klajn, J. Am. Chem. Soc. 2020, 142, 17721–17729.
- 56D. Samanta, J. Gemen, Z. Chu, Y. Diskin-Posner, L. J. Shimon, R. Klajn, Proc. Natl. Acad. Sci. U.S.A. 2018, 115, 9379–9384.
- 57J. Gemen, B. Stövesand, F. Glorius, B. J. Ravoo, Angew. Chem. Int. Ed. 2024, 63, e202413209.
- 58R. Gera, P. De, K. K. Singh, S. A. Jannuzzi, A. Mohanty, L. Velasco, Kulbir, P. K., J. Marco, K. Nagarajan, C. Pecharromen, P. M. Rodriguez-Pascual, S. DeBeer, D. Moonshiram, S. S. Gupta, J. Dasugupta, J. Am. Chem. Soc. 2024, 146, 21729–21741.
- 59M. Yoshizawa, Y. Takeyama, T. Okano, M. Fujita, J. Am. Chem. Soc. 2003, 125, 3243–3247.
- 60H. Takezawa, S. Akiba, T. Murase, M. Fujita, J. Am. Chem. Soc. 2015, 137, 7043–7046.
- 61H. Takezawa, K. Iizuka, M. Fujita, Angew. Chem. Int. Ed. 2024, 136, e202319140.
- 62K. Iizuka, H. Takezawa, M. Fujita, J. Am. Chem. Soc. 2023, 145, 25971–25975.
- 63 Deposition number CCDC-2403903 (for (G1)2⊂MC1) contain the supplementary crystallographic data for this paper. This data is provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum KarlsruheAccess Structures service.
- 64C. Bannwarth, S. Ehlert, S. Grimme, J. Chem. Theory Comput. 2019, 15, 1652–1671.
- 65H. Xu, T. K. Ronson, A. W. Heard, P. C. Teeuwen, L. Schneider, P. Pracht, J. D. Thoburn, D. J. Wales, J. R. Nitschke,Nat. Chem. 2025, 17, 1–8.
- 66C. Bannwarth, E. Caldeweyher, S. Ehlert, A. Hansen, P. Pracht, J. Seibert, S. Spicher, S. Grimme, Wiley Interdiscip. Rev. Comput. Mol. Sci. 2021, 11, e1493.
- 67H. Takezawa, K. Shitozawa, M. Fujita, Nat. Chem. 2020, 12, 574–578.
- 68T. Kusukawa, M. Yoshizawa, M. Fujita, Angew. Chem. Int. ed. Engl. 2001, 40, 1879–1884.
10.1002/1521-3773(20010518)40:10<1879::AID-ANIE1879>3.0.CO;2-I CAS PubMed Web of Science® Google Scholar
- 69M. Yoshizawa, N. Sato, M. Fujita, Chem. Lett. 2005, 34, 1392–1393.
- 70H. Takezawa, T. Murase, G. Resnati, P. Metrangolo, M. Fujita, J. Am. Chem. Soc. 2014, 136, 1786–1788.
- 71E. Ämmälahti, M. Bardet, D. Molko, J. Cadet, J. Magn. Reson., Ser A. 1996, 122, 230–232.
- 72P. Zhou, P. Li, Y. Zhao, K. Han, J. Phy. Chem. Lett. 2019, 10, 6929–6935.
- 73D. Sirbu, J. K. Karlsson, A. Harriman, J. Phy. Chem. A 2018, 122, 9160–9170.
- 74J. D. Kimball, S. Raut, L. P. Jameson, N. W. Smith, Z. Gryczynski, S. V. Dzyuba, RSC Adv. 2015, 5, 19508–19511.
- 75M.-H. Yu, X.-T. Liu, B. Space, Z. Chang, X.-H. Bu, Coord. Chem. Rev. 2021, 427, 213518.
- 76R. Gera, J. Dasgupta, Phy. Chem. Chem. Phy. 2021, 23, 9280–9284.
- 77A. Das, A. Jha, R. Gera, J. Dasgupta, J. Phy. Chem. C 2015, 119, 21234–21242.
- 78N. Nandi, K. Bhattacharyya, B. Bagchi, Chem. Rev. 2000, 100, 2013–2046.
- 79M. Yoshizawa, S. Miyagi, M. Kawano, K. Ishiguro, M. Fujita, J. Am. Chem. Soc. 2004, 126, 9172–9173.
- 80S. K. Pal, A. H. Zewail, Chem. Rev. 2004, 104, 2099–2124.
- 81D. Sun, Y. Wu, X. Han, S. Liu, Nat. Commun 2023, 14, 4190.
- 82R. Gera, A. Das, A. Jha, J. Dasgupta, J. Am. Chem. Soc. 2014, 136, 15909–15912.
- 83A. Das, I. Mandal, R. Venkatramani, J. Dasgupta, Sci. Adv. 2019, 5, eaav4806.
- 84Y. Furutani, H. Kandori, M. Kawano, K. Nakabayashi, M. Yoshizawa, M. Fujita, J. Am. Chem. Soc. 2009, 131, 4764–4768.
- 85P. Thordarson, Chem. Soc. Rev. 2011, 40, 1305–1323.
- 86K. Jeremy, J. Chem. Soc., Perkin Trans 1995, 1, 2231–2245.
- 87A. Rit, T. Pape, A. Hepp, F. E. Hahn, Organometallics 2011, 30, 334–347.
- 88N. Giuseppone, J. L. Schmitt, L. Allouche, J. M. Lehn, Angew. Chem. Int. Ed. 2008, 47, 2235–2239.
- 89W. Kabsch, Acta Crystallogr. D Biol. Crystallogr. 2010, 66, 125–132.
- 90W. Kabsch, Acta Crystallogr. D Biol. Crystallogr. 2010, 66, 133–144.
- 91G. M. Sheldrick, Acta Crystallogr. 2015, 71, 3–8.
- 92G. M. Sheldrick, Acta Cryst. C 2015, 71, 3–8.
- 93A. L. Spek, Acta Cryst. C 2015, 71, 9–18.