Enantioselective Palladium-Catalyzed α-Arylation of Acyclic Esters
Jianxun Huang
State Key Laboratory of Chemical Biology, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, 345 Ling Ling Road, Shanghai, 200032 China
Both authors contributed equally to this work.
Search for more papers by this authorDr. Chao Pei
Chemical & Analytical Development, Suzhou Novartis Technical Development Co., Ltd, Changshu, Jiangsu, 215537 China
Both authors contributed equally to this work.
Search for more papers by this authorCorresponding Author
Prof. Dr. He Yang
State Key Laboratory of Chemical Biology, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, 345 Ling Ling Road, Shanghai, 200032 China
E-mail: [email protected]; [email protected]; [email protected]
Search for more papers by this authorCorresponding Author
Dr. Bin Wu
Chemical & Analytical Development, Suzhou Novartis Technical Development Co., Ltd, Changshu, Jiangsu, 215537 China
E-mail: [email protected]; [email protected]; [email protected]
Search for more papers by this authorCorresponding Author
Prof. Dr. Wenjun Tang
State Key Laboratory of Chemical Biology, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, 345 Ling Ling Road, Shanghai, 200032 China
School of Chemistry and Materials Science, Hangzhou Institute for Advanced Study, University of Chinese Academy of Sciences, 1 Sub-lane Xiangshan, Hangzhou, 310024 China
E-mail: [email protected]; [email protected]; [email protected]
Search for more papers by this authorJianxun Huang
State Key Laboratory of Chemical Biology, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, 345 Ling Ling Road, Shanghai, 200032 China
Both authors contributed equally to this work.
Search for more papers by this authorDr. Chao Pei
Chemical & Analytical Development, Suzhou Novartis Technical Development Co., Ltd, Changshu, Jiangsu, 215537 China
Both authors contributed equally to this work.
Search for more papers by this authorCorresponding Author
Prof. Dr. He Yang
State Key Laboratory of Chemical Biology, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, 345 Ling Ling Road, Shanghai, 200032 China
E-mail: [email protected]; [email protected]; [email protected]
Search for more papers by this authorCorresponding Author
Dr. Bin Wu
Chemical & Analytical Development, Suzhou Novartis Technical Development Co., Ltd, Changshu, Jiangsu, 215537 China
E-mail: [email protected]; [email protected]; [email protected]
Search for more papers by this authorCorresponding Author
Prof. Dr. Wenjun Tang
State Key Laboratory of Chemical Biology, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, 345 Ling Ling Road, Shanghai, 200032 China
School of Chemistry and Materials Science, Hangzhou Institute for Advanced Study, University of Chinese Academy of Sciences, 1 Sub-lane Xiangshan, Hangzhou, 310024 China
E-mail: [email protected]; [email protected]; [email protected]
Search for more papers by this authorGraphical Abstract
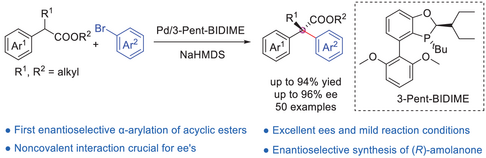
A Pd-catalyzed enantioselective α-arylation of α,α-disubstituted esters with aryl bromides is established for the first time by employing P-chiral monophosphorus ligand 3-Pent-BIDIME as a chiral ligand, leading to a series of enantioenriched α,α-diaryl esters possessing quaternary carbon stereocenters in moderate to good yields and high enantioselectivities. The method features a broad substrate scope, mild conditions, excellent functional group compatibility, and low Pd loadings (as low as 1 mol%). Its synthetic utilities are exemplified by the efficient preparation of a chiral α,α-diaryl substituted γ-lactone and the asymmetric synthesis of (R)-amolanone.
Abstract
A Pd-catalyzed enantioselective α-arylation of α,α-disubstituted esters with aryl bromides is established for the first time by employing P-chiral monophosphorus ligand 3-Pent-BIDIME as a chiral ligand, leading to a series of enantioenriched α,α-diaryl esters possessing quaternary carbon stereocenters in moderate to good yields and high enantioselectivities. The method features a broad substrate scope, mild conditions, excellent functional group compatibility, and low Pd loadings (as low as 1 mol%). The synthetic power of this protocol is exemplified by the efficient preparation of a chiral α,α-diaryl substituted γ-lactone and asymmetric synthesis of (R)-amolanone. DFT calculation revealed an NaBr-bridged downstream transmetallation and the importance of noncovalent interaction in controlling the enantioselectivity.
Conflict of Interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.
Supporting Information
| Filename | Description |
|---|---|
| anie202505458-sup-0001-SuppMat.pdf16 MB | Supporting Information |
| anie202505458-sup-0002-SuppMat.cif552.2 KB | Supporting Information |
| anie202505458-sup-0003-SuppMat.cif2.5 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1K. C. Nicolaou, Z. Yang, J. Liu, H. Ueno, P. G. Nantermet, R. K. Guy, C. F. Claiborne, J. Renaud, E. A. Couladouros, K. Paulvannan, E. J. Sorensen, Nature 1994. 367, 630–634.
- 2P. Yates, F. N. MacLachlan, I. D. Rae, M. Rosenberger, A. G. Szabo, C. R. Willis, M. P. Cava, M. Behforouz, M. V. Lakshmikantham, W. Zeiger, J. Am. Chem. Soc. 1973, 95, 7842–7850.
- 3K. W. Quasdorf, L. E. Overman, Nature 2014, 516, 181–191.
- 4C. R. Jamison, J. J. Badillo, J. M. Lipshultz, R. J. Comito, D. W. C. MacMillan, Nat. Chem. 2017, 9, 1165–1169.
- 5Y. Ping, Y. Li, J. Zhu, W. Kong, Angew. Chem. Int. Ed. 2019, 58, 1562; Angew. Chem. 2019, 131, 1576.
- 6Y. Que, H. Shao, H. He, S. Gao, Angew. Chem. Int. Ed. 2020, 59, 7444–7449; Angew. Chem. 2020, 132, 7514.
- 7C. Li, S. S. Ragab, G. Liu, W. Tang, Nat. Prod. Rep. 2020, 37, 276–292.
- 8E. Posey, J. Leonard, J. A. Bargen, W. H. Dearing, C. F. Code, Gastroenterology 1948, 11, 56.
- 9W. W. Arthur, W. B. Brownell, J. Am. Chem. Soc. 1952, 74, 653.
- 10A. Willeford, J. S. Enciso, Pharmacotherapy 2023, 43, 1397–1404.
- 11M. W. Baines, J. E. Davies, D. N. Kellett, P. L. Munt, J. Int. Med. Res. 1976, 4, 5.
- 12R. C. Heel, R. N. Brogden, T. M. Speight, G. S. Avery, Drugs 1978, 15, 331–368.
- 13P. J. Meffin, E. W. Robert, R. A. Winkle, S. Harapat, F. A. Peters, D. C. Harrison, J. Pharmacokinet. Biopharm. 1979, 7, 29.
- 14J.r. P. Danilo, M. R. Rosen, Am. Hear. J. 1976, 92, 532.
- 15C. E. Gidding, S. J. Kellie, W. A. Kamps, S. S. de Graaf, Crit. Rev. Oncol. Hematol. 1999, 29, 267–287.
- 16S. S. Legha, Med. Toxicol. 1986, 1, 421–427.
- 17K. Aley, D. Reichling, J. Levine, Neuroscience 1996, 73, 259–265.
- 18V. S. Sethi, D. V. Jackson Jr., D. R. White, F. Richards, J. J. Stuart, H. B. Muss, Cancer Res. 1981, 41, 3551.
- 19J. Nakagawa, T. Takahata, Y. Chen, Cancer Chemother. Pharmacol. 2023, 92, 391.
- 20For selected references: R. Jiang, L. Ding, C. Zheng, S.-L. You, Science 2021, 371, 380–386.
- 21Z. Wang, Z.-P. Yang, G. C. Fu, Nat. Chem. 2021, 13, 236–242.
- 22C. J. Douglas, L. E. Overmann, Proc. Nat. Acad. Sci. USA 2004, 101, 5363–5367.
- 23Y.-J. Hao, X.-S. Hu, Y. Zhou, J. Zhou, J.-S. Yu, ACS Catal. 2020, 10, 955–993.
- 24J. García-Fortanet, S. L. Buchwald, Angew. Chem. Int. Ed. 2008, 47, 8108; Angew. Chem. 2008, 120, 8228.
- 25P. Nareddy, L. Mantilli, L. Guénée, C. Mazet, Angew. Chem. Int. Ed. 2012, 51, 3826; Angew. Chem. 2012, 124, 3892–3897.
- 26S. Lee, J. F. Hartwig, J. Org. Chem. 2001, 66, 3402–3415.
- 27T. Arao, K. Kondo, T. Aoyama, Tetrahedron Lett. 2006, 47, 1417.
- 28E. P. Kündig, T. M. Seidel, Y.-X. Jia, G. Bernardinelli, Angew. Chem. Int. Ed. 2007, 46, 8484; Angew. Chem. 2007, 119, 8636.
- 29Y.-X. Jia, M. Hillgren, E. L. Watson, S. P. Marsden, E. P. Kündig, Chem. Commun. 2008, 44, 4040.
- 30Y.-X. Jia, D. Katayev, G. Bernardinelli, T. M. Seidel, E. P. Kündig, Chem. - Eur. J. 2010, 16, 6300–6309.
- 31X. Luan, R. Mariz, C. Robert, M. Gatti, S. Blumentritt, A. Linden, R. Dorta, Org.Lett 2008, 10, 5569–5572.
- 32X. Luan, L. Wu, E. Drinkel, R. Mariz, M. Gatti, R. Dorta, Org. Lett. 2010, 12, 1912–1915.
- 33L. Wu, L. Falivene, E. Drinkel, S. Grant, A. Linden, L. Cavallo, R. Dorta, Angew. Chem. Int. Ed. 2012, 51, 2870 Angew. Chem. 2012, 124, 2924–2927.
- 34S. Würtz, C. Lohre, R. Fröhlich, K. Bergander, F. Glorius, J. Am. Chem. Soc. 2009, 131, 8344–8345.
- 35W. He, W. Zhao, B. Zhou, H. Liu, X. Li, L. Li, J. Li, J. Shi, Molecules 2016, 21, 742.
- 36T. Arao, K. Kondo, T. Aoyama, Chem. Pharm. Bull. 2006, 54, 1743–1744.
- 37T. Wu, Q. Zhou, W. Tang, Angew. Chem. Int. Ed. 2021, 60, 9978; Angew. Chem. 2021, 133, 10066.
- 38T. Wu, X. Kang, H. Bai, W. Xiong, G. Xu, W. Tang, Org. Lett. 2020, 22, 4602–4607.
- 39J. Åhman, J. P. Wolfe, M. V. Troutman, M. Palucki, S. L. Buchwald, J. Am. Chem. Soc. 1998, 120, 1918.
- 40T. Hamada, A. Chieffi, J. Åhman, S. L. Buchwald, J. Am. Chem. Soc. 2002, 124, 1261–1268.
- 41X. Liao, Z. Weng, J. F. Hartwig, J. Am. Chem. Soc. 2008, 130, 195–200.
- 42S. Ge, J. F. Hartwig, J. Am. Chem. Soc. 2011, 133, 16330–16333.
- 43J. Cornella, E. P. Jackson, R. Martin, Angew. Chem. Int. Ed. 2015, 54, 4075–4078; Angew. Chem. 2015, 127, 4147.
- 44X. Rao, N. Li, H. Bai, C. Dai, Z. Wang, W. Tang, Angew. Chem. Int. Ed. 2018, 57, 12328; Angew. Chem. 2018, 130, 12508.
- 45J. Guo; L. Lin, Y. Liu, X. Li, X. Liu, X. Feng, Org. Lett. 2016, 18, 5540–5543.
- 46M. Orlandi, G. Licini, J. Org. Chem. 2020, 85, 11511–11518.
- 47D. J. Spielvogel, S. L. Buchwald, J. Am. Chem. Soc. 2002, 124, 3500–3501.
- 48J. Guo; S. Dong; Y. Zhang, Y. Kuang, X. Liu, L. Lin, X. Feng, Angew. Chem. Int. Ed. 2013, 52, 10245–10249; Angew. Chem. 2013, 125, 10435.
- 49A. M. Taylor, R. A. Altman, S. L. Buchwald, J. Am. Chem. Soc. 2009, 131, 9900–9901.
- 50T. Liu, J. Feng, C. Chen, Z. Deng, R. Kotagiri, G. Zhou, X. Zhang, Q. Cai, Org. Lett. 2019, 21, 4505–4509.
- 51Y. Jin, M. Chen, S. Ge, J. F. Hartwig, Org. Lett. 2017, 19, 1390–1393.
- 52S. Qi, W. Ye, Y. Hua, L. Pan, J. Yang, J. Zhang, Nat. Synth. 2023, 3, 357.
- 53C. I. Jette, I. Geibei, S. Bachman, M. Hayashi, S. Sakurai, H. Shimizu, J. B. Morgan, B. M. Stoltz, Angew. Chem. Int. Ed. 2019, 58, 4297; Angew. Chem. 2019, 131, 4341.
- 54E. A. Bercot, S. Caille, T. M. Bostick, K. Ranganathan, R. Jensen, M. M. Faul, Org. Lett. 2008, 10, 5251.
- 55Z. Jiao, K. W. Chee, J. S. Zhou, J. Am.Chem. Soc. 2016, 138, 16240–16243.
- 56Z. Pan, W. Li, S. Zhu, F. Liu, H.-H. Wu, J. Zhang, Angew. Chem. Int. Ed. 2021, 60, 18542–18546; Angew. Chem. 2021, 133, 18690.
- 57X. Xie, Y. Chen, D. Ma, J. Am. Chem. Soc. 2006, 128, 16050–16051.
- 58R. Zhang, Q. Zhou, X. Wang, L. Xu, D. Ma, Angew. Chem. Int. Ed. 2023, 62, e202312383; Angew. Chem. 2023, 135, e202312383.
- 59M. Simonetti, D. M. Cannas, X. Just-Baringo, I. J. Vitorica-Yrezabal, I. Larrosa, Nat. Chem. 2018, 10, 724–731.
- 60Z. Wang, S. Chen, C. Chen, Y. Yang, C. Wang, Angew. Chem. Int. Ed. 2023, 62, e202215963; Angew. Chem. 2023, 135, e202215963.
- 61 Deposition Numbers 2444586 (for 3ag) and 2416778 (for Pd complex A) contain the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service.
- 62A. F. da Silva, M. A. S. Afonso, R. A. Cormanich, I. D. Jurberg, Chem. - Eur. J. 2020, 26, 5648–5653.
- 63R. K. Gordon, F. N. Padilla, E. Moore, B. P. Doctor, P. K. Chiang, Biochem. Pharmacol. 1983, 32, 2979–2981.
- 64R. F. Genovese, T. F. Elsmore, J. M. Witkin, Pharmacol. Biochem. Behav. 1990, 37, 117–122.
- 65W. Fu, W. Tang, ACS Catal. 2016, 6, 4814–4858.
- 66G. Xu, C. H. Senanayake, W. Tang, Acc. Chem. Res. 2019, 52, 1101–1112.
- 67T. Imamoto, Chem. Rev. 2024, 124, 8657–8739.