Dimensionality Reduction of Metal–Organic Frameworks to Monolayers for Enhanced Electrocatalysis
Graphical Abstract
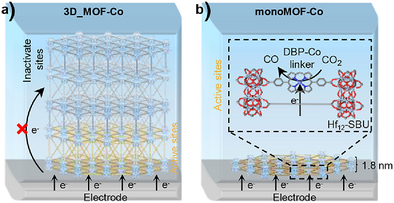
Dimensionality reduction of 3D MOFs into 1.8-nm-thick monoMOF maximizes catalytic site exposure and enhances CO2 electroreduction. The cobalt(II)–porphyrin-embedded monoMOF-Co achieves 93% CO Faradaic efficiency (versus 51% for 3D_MOF-Co) at −0.8 V versus RHE, with a 10,600 h−1 turnover frequency and 47-hour stability in near-neutral electrolyte, demonstrating superior electron/mass transfer through dimensional engineering.
Abstract
Metal–organic frameworks (MOFs) are potential candidates for electrocatalysis due to their well-defined, tunable structures, and ability to incorporate diverse active sites. However, their inherent insulating nature restricts electron transfer from electrode to remote active sites, leading to diminished catalytic performance. In this work, we present a novel strategy to overcome this limitation by reducing 3D MOFs (3D_MOFs) into monolayered MOFs (monoMOFs) with a thickness of ∼1.8 nm, maximizing the exposure of catalytic sites to the electrode and enhancing electrocatalytic performance. We designed and synthesized a monoMOF incorporating cobalt(II)–porphyrin sites in the linker (monoMOF-Co) for CO2 electroreduction. After being grafted onto graphene oxide, the monoMOF-Co exhibited a peak faradaic efficiency for CO production (FECO = 93%), surpassing the performance of a 3D_MOF incorporating the same porphyrin–Co-based linker (3D_MOF-Co, FECO = 51%). Additionally, monoMOF-Co achieved a turnover frequency of 10 600 h−1 at −0.8 V versus the reversible hydrogen electrode (RHE) and maintained stability over 47 h in a near-neutral aqueous solution. In situ spectroscopic studies further confirmed the distinct electric field environment in the Stern layer between monoMOF-Co and 3D_MOF-Co. Furthermore, similar enhancement effects of monoMOFs over 3D_MOFs were observed in the nitrate and oxygen electroreduction reactions, highlighting the broader applicability of monoMOFs in electrocatalysis.
Conflict of Interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.