DNA Nanotubule-Based Nanodevices with ATP-Responsive Gating for Direct Cytosolic Delivery of Nucleic Acids and Proteins
Di Gao
State Key Laboratory of Analytical Chemistry for Life Science, School of Chemistry and Chemical Engineering, Nanjing University, Nanjing, 210023 China
Both authors contributed equally to this work.
Search for more papers by this authorZiqi Xu
State Key Laboratory of Analytical Chemistry for Life Science, School of Chemistry and Chemical Engineering, Nanjing University, Nanjing, 210023 China
Collaborative Innovation Center of Biomedical Functional Materials and Key Laboratory of Biofunctional Materials of Jiangsu Province, School of Chemistry and Materials Science, Nanjing Normal University, Nanjing, 210023 China
Both authors contributed equally to this work.
Search for more papers by this authorXiangli Li
State Key Laboratory of Analytical Chemistry for Life Science, School of Chemistry and Chemical Engineering, Nanjing University, Nanjing, 210023 China
Search for more papers by this authorYuhan Zhao
State Key Laboratory of Analytical Chemistry for Life Science, School of Chemistry and Chemical Engineering, Nanjing University, Nanjing, 210023 China
Search for more papers by this authorQianhao Min
State Key Laboratory of Analytical Chemistry for Life Science, School of Chemistry and Chemical Engineering, Nanjing University, Nanjing, 210023 China
Search for more papers by this authorZixuan Chen
State Key Laboratory of Analytical Chemistry for Life Science, School of Chemistry and Chemical Engineering, Nanjing University, Nanjing, 210023 China
Search for more papers by this authorQin Xu
Institute of Innovation Materials and Energy, School of Chemistry and Chemical Engineering, Yangzhou University, Yangzhou, 225002 China
Search for more papers by this authorCorresponding Author
Ye Tian
State Key Laboratory of Analytical Chemistry for Life Science, School of Chemistry and Chemical Engineering, Nanjing University, Nanjing, 210023 China
E-mail: [email protected]; [email protected]; [email protected]
Search for more papers by this authorCorresponding Author
Junpeng Xu
State Key Laboratory of Analytical Chemistry for Life Science, School of Chemistry and Chemical Engineering, Nanjing University, Nanjing, 210023 China
State Key Laboratory of Pharmaceutical Biotechnology, Medicine School, Nanjing University, Nanjing, 210093 China
E-mail: [email protected]; [email protected]; [email protected]
Search for more papers by this authorCorresponding Author
Jun-Jie Zhu
State Key Laboratory of Analytical Chemistry for Life Science, School of Chemistry and Chemical Engineering, Nanjing University, Nanjing, 210023 China
E-mail: [email protected]; [email protected]; [email protected]
Search for more papers by this authorDi Gao
State Key Laboratory of Analytical Chemistry for Life Science, School of Chemistry and Chemical Engineering, Nanjing University, Nanjing, 210023 China
Both authors contributed equally to this work.
Search for more papers by this authorZiqi Xu
State Key Laboratory of Analytical Chemistry for Life Science, School of Chemistry and Chemical Engineering, Nanjing University, Nanjing, 210023 China
Collaborative Innovation Center of Biomedical Functional Materials and Key Laboratory of Biofunctional Materials of Jiangsu Province, School of Chemistry and Materials Science, Nanjing Normal University, Nanjing, 210023 China
Both authors contributed equally to this work.
Search for more papers by this authorXiangli Li
State Key Laboratory of Analytical Chemistry for Life Science, School of Chemistry and Chemical Engineering, Nanjing University, Nanjing, 210023 China
Search for more papers by this authorYuhan Zhao
State Key Laboratory of Analytical Chemistry for Life Science, School of Chemistry and Chemical Engineering, Nanjing University, Nanjing, 210023 China
Search for more papers by this authorQianhao Min
State Key Laboratory of Analytical Chemistry for Life Science, School of Chemistry and Chemical Engineering, Nanjing University, Nanjing, 210023 China
Search for more papers by this authorZixuan Chen
State Key Laboratory of Analytical Chemistry for Life Science, School of Chemistry and Chemical Engineering, Nanjing University, Nanjing, 210023 China
Search for more papers by this authorQin Xu
Institute of Innovation Materials and Energy, School of Chemistry and Chemical Engineering, Yangzhou University, Yangzhou, 225002 China
Search for more papers by this authorCorresponding Author
Ye Tian
State Key Laboratory of Analytical Chemistry for Life Science, School of Chemistry and Chemical Engineering, Nanjing University, Nanjing, 210023 China
E-mail: [email protected]; [email protected]; [email protected]
Search for more papers by this authorCorresponding Author
Junpeng Xu
State Key Laboratory of Analytical Chemistry for Life Science, School of Chemistry and Chemical Engineering, Nanjing University, Nanjing, 210023 China
State Key Laboratory of Pharmaceutical Biotechnology, Medicine School, Nanjing University, Nanjing, 210093 China
E-mail: [email protected]; [email protected]; [email protected]
Search for more papers by this authorCorresponding Author
Jun-Jie Zhu
State Key Laboratory of Analytical Chemistry for Life Science, School of Chemistry and Chemical Engineering, Nanjing University, Nanjing, 210023 China
E-mail: [email protected]; [email protected]; [email protected]
Search for more papers by this authorGraphical Abstract
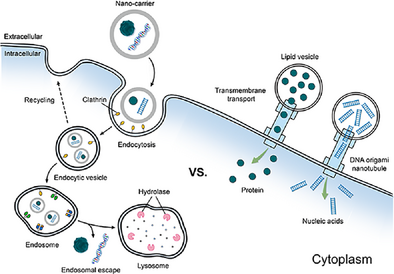
Schematic illustration of two pathways for macromolecular therapeutics delivery: nanoparticle-adopted endocytosis (left) and DNA nanotubule-mediated cytosolic delivery (right). By bypassing conventional endocytic routes, the nanotubules directly transport substantial payloads, including nucleic acids and proteins, across the plasma membrane.
Abstract
Delivering biomacromolecules to the cytosol remains a formidable challenge, as these molecules are predominantly sequestered within endosomes after endocytosis. The limited efficacy of current delivery systems in promoting reliable endosomal escape underscores the need for innovative strategies. Here, we report a DNA origami nanotubule to construct transmembrane delivery nanodevices with size-selective gating and ATP-responsive channel activation. By integrating unilamellar vesicles as large storage compartments, these nanodevices can encapsulate a wide range of macromolecules, including small interfering RNA, messenger RNA, plasmid DNA, and CRISPR-Cas9 ribonucleoprotein complexes. By bypassing traditional endocytic pathways, the nanotubules enable the delivery of substantial payload quantities directly across the plasma membrane. This approach provides a promising platform for delivering macromolecular therapeutics into the cytosol, advancing gene therapy strategies, and broadening their biomedical applications.
Conflict of Interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Supporting Information
| Filename | Description |
|---|---|
| anie202505290-sup-0001-SuppMat.docx52.9 MB | Supporting Information |
| anie202505290-sup-0002-Movie1.mp4194.2 KB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1S. F. Dowdy, Nat. Biotechnol. 2017, 35, 222–229.
- 2J. P. Luzio, P. R. Pryor, N. A. Bright, Nat. Rev. Mol. Cell Biol. 2007, 8, 622–632.
- 3J. J. Rennick, A. P. R. Johnston, R. G. Parton, Nat. Nanotechnol. 2021, 16, 266–276.
- 4K. O'Brien, K. Breyne, S. Ughetto, L. C. Laurent, X. O. Breakefield, Nat. Rev. Mol. Cell Biol. 2020, 21, 585–606.
- 5A. M. Vargason, A. C. Anselmo, S. Mitragotri, Nat. Biomed. Eng. 2021, 5, 951–967.
- 6X. Huang, N. Kong, X. Zhang, Y. Cao, R. Langer, W. Tao, Nat. Med. 2022, 28, 2273–2287.
- 7J. Gilleron, W. Querbes, A. Zeigerer, A. Borodovsky, G. Marsico, U. Schubert, K. Manygoats, S. Seifert, C. Andree, M. Stöter, H. Epstein-Barash, L. Zhang, V. Koteliansky, K. Fitzgerald, E. Fava, M. Bickle, Y. Kalaidzidis, A. Akinc, M. Maier, M. Zerial, Nat. Biotechnol. 2013, 31, 638–646.
- 8A. Wittrup, A. Ai, X. Liu, P. Hamar, R. Trifonova, K. Charisse, M. Manoharan, T. Kirchhausen, J. Lieberman, Nat. Biotechnol. 2015, 33, 870–876.
- 9S. A. Dilliard, D. J. Siegwart, Nat. Rev. Mater. 2023, 8, 282–300.
- 10K. A. Hajj, K. A. Whitehead, Nat. Rev. Mater. 2017, 2, 17056.
- 11I. Lostalé-Seijo, J. Montenegro, Nat. Rev. Chem. 2018, 2, 258–277.
- 12B. B. Mendes, J. Conniot, A. Avital, D. B. Yao, X. Y. Jiang, X. Zhou, N. Sharf-Pauker, Y. L. Xiao, O. Adir, H. J. Liang, J. J. Shi, A. Schroeder, J. Conde, Nat. Rev. Methods Primers 2022, 2, 25.
- 13L. I. Selby, C. M. Cortez-Jugo, G. K. Such, A. P. R. Johnston, Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2017, 9, e1452.
- 14Y. Sun, S. Y. Lau, Z. W. Lim, S. C. Chang, F. Ghadessy, A. Partridge, A. Miserez, Nat. Chem. 2022, 14, 274–283.
- 15Y. Zhao, J. Qin, D. Yu, Y. Liu, D. Song, K. Tian, H. Chen, Q. Ye, X. Wang, T. Xu, H. Xuan, N. Sun, W. Ma, J. Zhong, P. Sun, Y. Song, J. Hu, Y. Zhao, X. Hou, X. Meng, C. Jiang, J. Cai, Nat. Nanotechnol. 2024, 19, 1869–1879.
- 16Y. Zhuo, Z. Luo, Z. Zhu, J. Wang, X. Li, Z. Zhang, C. Guo, B. Wang, D. Nie, Y. Gan, G. Hu, M. Yu, Nat. Nanotechnol. 2024, 19, 1858.
- 17S. Du, S. S. Liew, L. Li, S. Q. Yao, J. Am. Chem. Soc. 2018, 140, 15986-15996.
- 18J. Kreitz, M. J. Friedrich, A. Guru, B. Lash, M. Saito, R. K. Macrae, F. Zhang, Nature 2023, 616, 357–364.
- 19W. Tai, P. Zhao, X. Gao, Sci. Adv. 2020, 6, eabb0310.
- 20S. Mantri, K. Tanuj Sapra, S. Cheley, T. H. Sharp, H. Bayley, Nat. Commun. 2013, 4, 1725.
- 21S. Maeda, S. Nakagawa, M. Suga, E. Yamashita, A. Oshima, Y. Fujiyoshi, T. Tsukihara, Nature 2009, 458, 597–602.
- 22Y. Xing, A. Rottensteiner, J. Ciccone, S. Howorka, Angew. Chem. Int. Ed. 2023, 62, e202303103.
- 23C. Lv, X. Gu, H. Li, Y. Zhao, D. Yang, W. Yu, D. Han, J. Li, W. Tan, ACS Nano 2020, 14, 14616–14626.
- 24Y. Li, C. Maffeo, H. Joshi, A. Aksimentiev, B. Ménard, R. Schulman, Sci. Adv. 2022, 8, eabq4834.
- 25R. P. Thomsen, M. G. Malle, A. H. Okholm, S. Krishnan, S. S. R. Bohr, R. S. Sorensen, O. Ries, S. Vogel, F. C. Simmel, N. S. Hatzakis, J. Kjems, Nat. Commun. 2019, 10, 5655.
- 26R. Tenchov, R. Bird, A. E. Curtze, Q. Zhou, ACS Nano 2021, 15, 16982–17015.
- 27J. I. Cutler, E. Auyeung, C. A. Mirkin, J. Am. Chem. Soc. 2012, 134, 1376–1391.
- 28C. H. Lin, D. J. Patei, Chem. Biol. 1997, 4, 817–832.
- 29E. Dulkeith, M. Ringler, T. A. Klar, J. Feldmann, A. Muñoz Javier, W. J. Parak, Nano Lett. 2005, 5, 585–589.
- 30M. Hu, C. Yuan, T. Tian, X. Wang, J. Sun, E. Xiong, X. Zhou, J. Am. Chem. Soc. 2020, 142, 7506–7513.
- 31P. Juin, O. Geneste, F. Gautier, S. Depil, M. Campone, Nat. Rev. Cancer 2013, 13, 455–465.
- 32P. D. Hsu, E. S. Lander, F. Zhang, Cell 2014, 157, 1262–1278.
- 33T. Wan, D. Niu, C. Wu, F.-J. Xu, G. Church, Y. Ping, Mater. Today 2019, 26, 40–66.
- 34S. Tong, B. Moyo, C. M. Lee, K. Leong, G. Bao, Nat. Rev. Mater. 2019, 4, 726–737.
- 35N. C. Seeman, H. F. Sleiman, Nat. Rev. Mater. 2017, 3, 17068.
- 36Y. Ding, J. Liu, J. Am. Chem. Soc. 2023, 145, 7540–7547.
- 37Y. Z. Xing, A. Dorey, L. Jayasinghe, S. Howorka, Nat. Nanotechnol. 2022, 17, 708–713.
- 38J. R. Burns, E. Stulz, S. Howorka, Nano Lett. 2013, 13, 2351–2356.
- 39J. R. Burns, K. Göpfrich, J. W. Wood, V. V. Thacker, E. Stulz, U. F. Keyser, S. Howorka, Angew. Chem. Int. Ed. 2013, 52, 12069–12072.
- 40M. You, Y. Lyu, D. Han, L. Qiu, Q. Liu, T. Chen, C. Sam Wu, L. Peng, L. Zhang, G. Bao, W. Tan, Nat. Nanotechnol. 2017, 12, 453.
- 41S. Krishnan, D. Ziegler, V. Arnaut, T. G. Martin, K. Kapsner, K. Henneberg, A. R. Bausch, H. Dietz, F. C. Simmel, Nat. Commun. 2016, 7, 12787.
- 42A. Fragasso, N. De Franceschi, P. Stömmer, E. O. van der Sluis, H. Dietz, C. Dekker, ACS Nano 2021, 15, 12768–12779.