ATP-Assisted Electron and Proton Transfer Boosting Redox Metabolism-Induced Ferroptosis and Apoptosis for Cancer Therapy
Shangjie An
State Key Laboratory of Electroanalytical Chemistry, Changchun Institute of Applied Chemistry, Chinese Academy of Sciences, Changchun, Jilin, 130022 China
School of Applied Chemistry and Engineering, University of Science and Technology of China, Hefei, Anhui, 230026 China
Search for more papers by this authorWenyao Zhen
State Key Laboratory of Electroanalytical Chemistry, Changchun Institute of Applied Chemistry, Chinese Academy of Sciences, Changchun, Jilin, 130022 China
School of Applied Chemistry and Engineering, University of Science and Technology of China, Hefei, Anhui, 230026 China
Search for more papers by this authorYue Wang
Research Center for Analytical Science, College of Chemistry, Nankai University, Tianjin, 300071 China
Search for more papers by this authorXiaodan Jia
Research Center for Analytical Science, College of Chemistry, Nankai University, Tianjin, 300071 China
Search for more papers by this authorCorresponding Author
Xiue Jiang
State Key Laboratory of Electroanalytical Chemistry, Changchun Institute of Applied Chemistry, Chinese Academy of Sciences, Changchun, Jilin, 130022 China
School of Applied Chemistry and Engineering, University of Science and Technology of China, Hefei, Anhui, 230026 China
Research Center for Analytical Science, College of Chemistry, Nankai University, Tianjin, 300071 China
E-mail: [email protected]
Search for more papers by this authorShangjie An
State Key Laboratory of Electroanalytical Chemistry, Changchun Institute of Applied Chemistry, Chinese Academy of Sciences, Changchun, Jilin, 130022 China
School of Applied Chemistry and Engineering, University of Science and Technology of China, Hefei, Anhui, 230026 China
Search for more papers by this authorWenyao Zhen
State Key Laboratory of Electroanalytical Chemistry, Changchun Institute of Applied Chemistry, Chinese Academy of Sciences, Changchun, Jilin, 130022 China
School of Applied Chemistry and Engineering, University of Science and Technology of China, Hefei, Anhui, 230026 China
Search for more papers by this authorYue Wang
Research Center for Analytical Science, College of Chemistry, Nankai University, Tianjin, 300071 China
Search for more papers by this authorXiaodan Jia
Research Center for Analytical Science, College of Chemistry, Nankai University, Tianjin, 300071 China
Search for more papers by this authorCorresponding Author
Xiue Jiang
State Key Laboratory of Electroanalytical Chemistry, Changchun Institute of Applied Chemistry, Chinese Academy of Sciences, Changchun, Jilin, 130022 China
School of Applied Chemistry and Engineering, University of Science and Technology of China, Hefei, Anhui, 230026 China
Research Center for Analytical Science, College of Chemistry, Nankai University, Tianjin, 300071 China
E-mail: [email protected]
Search for more papers by this authorGraphical Abstract
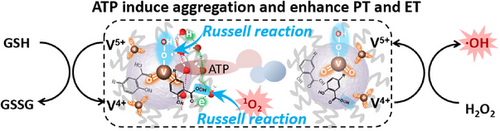
Vanadium and aurintricarboxylic coordination nanoparticles generate singlet oxygen and hydroxyl radicals specifically triggered by acidic H2O2 via dual-Russell reaction and Fenton-like reaction while depleting glutathione. Significantly, adenosine triphosphate induces VAP NPs aggregation, strengthens hydrogen-bonding network, and accelerates proton/electron transfer, thereby amplifying ROS level to co-activate ferroptosis and apoptosis.
Abstract
Compared to the intractability of traditional apoptosis, the vulnerability exposed by cancer cell metabolic reprogramming provides an advantage for ferroptosis treatment. Herein, we developed vanadate and aurintricarboxylic acid coordination nanoparticles (VAP NPs) that synergistically trigger dual cell death pathways. This nanoplatform leveraged dual-Russell mechanisms and Fenton reactions to generate singlet oxygen/hydroxyl radicals in the tumor microenvironment (TME) while depleting glutathione via vanadium redox cycling, thereby silencing glutathione peroxidase 4 and modulating the Kelch-like ECH-associated protein 1 (KEAP1)/nuclear factor erythroid 2-related factor 2 (NRF2)/heme oxygenase 1 (HMOX1) axis. Notably, TME-overexpressed adenosine triphosphate (ATP) acted as a biochemical catalyst, accelerating the transfer of protons and electrons during reactive oxygen species generation to amplify therapeutic efficacy. Therefore, VAP NPs could achieve outstanding efficacy for intrinsically stimulated synergy of ferroptosis and apoptosis in tumor therapy. This study provides reference for revealing the new function of ATP in enhancing the regulation of redox metabolism.
Conflict of Interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Supporting Information
| Filename | Description |
|---|---|
| anie202504542-sup-0001-SuppMat.docx11.1 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1B. R. Stockwell, Cell 2022, 185, 2401–2421.
- 2B. R. Stockwell, J. P. F. Angeli, H. Bayir, A. I. Bush, M. Conrad, S. J. Dixon, S. Fulda, S. Gascon, S. K. Hatzios, V. E. Kagan, K. Noel, X. Jiang, A. Linkermann, M. E. Murphy, M. Overholtzer, A. Oyagi, G. C. Pagnussat, J. Park, Q. Ran, C. S. Rosenfeld, K. Salnikow, D. Tang, F. M. Torti, S. V. Torti, S. Toyokuni, K. A. Woerpel, D. D. Zhang, Cell 2017, 171, 273–285.
- 3X. Chen, R. Kang, G. Kroemer, D. Tang, Nat. Rev. Clin. Oncol. 2021, 18, 280–296.
- 4N. N. Pavlova, J. Zhu, C. B. Thompson, Cell Metab. 2022, 34, 355–377.
- 5Z. Li, B. W. Ji, P. D. Dixit, K. Tchourine, E. C. Lien, A. M. Hosios, K. L. Abbott, J. C. Rutter, A. M. Westermark, E. F. Gorodetsky, L. B. Sullivan, M. G. Vander Heiden, D. Vitkup, Nat. Metab. 2022, 4, 711–723.
- 6P. Swietach, E. Boedtkjer, S. F. Pedersen, Nat. Rev. Cancer 2023, 23, 825–841.
- 7R. A. Cairns, I. S. Harris, T. W. Mak, Nat. Rev. Cancer 2011, 11, 85–95.
- 8B. Faubert, A. Solmonson, R. J. DeBerardinis, Science 2020, 368, 152.
- 9C. Schiliro, B. L. Firestein, Cells 2021, 10, 1056.
- 10Y. Wang, X. Jia, S. An, W. Yin, J. Huang, X. Jiang, Adv. Mater. 2024, 36, 2301810.
- 11Y. J. Li, C. Zhang, A. Martincuks, A. Herrmann, H. Yu, Nat. Rev. Cancer 2023, 23, 115–134.
- 12A. Karlstaedt, J. Moslehi, R. A. de Boer, Nat. Rev. Cardiol. 2022, 19, 414–425.
- 13G. Lei, L. Zhuang, B. Gan, Nat. Rev. Cancer 2022, 22, 381–396.
- 14S. Doll, B. Proneth, Y. Y. Tyurina, E. Panzilius, S. Kobayashi, I. IngoId, M. Irmler, J. Beckers, M. Aichler, A. Walch, H. Prokisch, D. Trümbach, G. Mao, F. Qu, H. Bayir, J. Füllekrug, C. H. Scheel, W. Wurst, J. A. Schick, V. E. Kagan, J. P. F. Angeli, M. Conrad, Nat. Chem. Biol. 2017, 13, 91–98.
- 15C. Liang, X. Zhang, M. Yang, X. Dong, Adv. Mater. 2019, 31, 1904197.
- 16K. Liu, L. Huang, S. Qi, S. Liu, W. Xie, L. Du, J. Cui, X. Zhang, B. Zhang, L. Liu, D. Li, H. Sun, Adv. Healthcare Mater. 2023, 12, 2203085.
- 17Q. Liu, Y. Zhao, H. Zhou, C. Chen, Regener. Biomater. 2023, 10, rbad004.
- 18D. Xiang, L. Zhou, R. Yang, F. Yuan, Y. Xu, Y. Yang, Y. Qiao, X. Li, Int. J. Nanomed. 2024, 19, 2091–2112.
- 19X. Jiang, B. R. Stockwell, M. Conrad, Nat. Rev. Mol. Cell Biol. 2021, 22, 266–282.
- 20S. V. Torti, F. M. Torti, Nat. Rev. Cancer 2013, 13, 342–355.
- 21X. Zhang, L. Wu, W. Zhen, S. Li, X. Jiang, Fundam. Res. 2022, 2, 66–73.
- 22Z. Li, L. Chen, C. Chen, Y. Zhou, D. Hu, J. Yang, Y. Chen, W. Zhuo, M. Mao, X. Zhang, L. Xu, L. Wang, J. Zhou, Biomark. Res. 2020, 8, 58.
- 23A. B. Cook, P. Decuzzi, ACS Nano 2021, 15, 2068–2098.
- 24N. Kang, S. Son, S. Min, H. Hong, C. Kim, J. An, J. S. Kim, H. Kang, Chem. Soc. Rev. 2023, 52, 3955–3972.
- 25W. Zhen, S. An, S. Wang, W. Hu, Y. Li, X. Jiang, J. Li, Adv. Mater. 2021, 33, 2101572.
- 26M. S. Nakazawa, B. Keith, M. C. Simon, Nat. Rev. Cancer 2016, 16, 663–673.
- 27W. R. Wilson, M. P. Hay, Nat. Rev. Cancer 2011, 11, 393–410.
- 28Y. Dai, C. Xu, X. Sun, X. Chen, Chem. Soc. Rev. 2017, 46, 3830–3852.
- 29S. Kwon, H. Ko, D. G. You, K. Kataoka, J. H. Park, Acc. Chem. Res. 2019, 52, 1771–1782.
- 30Z. Ge, S. Liu, Chem. Soc. Rev. 2013, 42, 7289–7325.
- 31Y. Xu, H. Wang, Z. Qiao, Chin. J. Chem. 2022, 40, 2815–2824.
- 32S. Xie, Z. Wang, T. Fu, L. Zheng, H. Wu, L. He, H. Huang, C. Yang, R. Wang, X. Qian, L. Qiu, W. Tan, Angew. Chem. Int. Ed. 31 2021, 61, e20220122.
- 33Z. Zhou, Y. Liu, M. Zhang, C. Li, R. Yang, J. Li, C. Qian, M. Sun, Adv. Funct. Mater. 2019, 29, 1904144.
- 34F. Liu, J. Zhu, P. Dai, J. Deng, J. Qin, Z. Yuchi, A. Fan, Z. Wang, Y. Zhao, Adv. Funct. Mater. 2021, 31, 2009157.
- 35N. Druzhyna, B. Szczesny, G. Olah, K. Modis, A. Asimakopoulou, A. Pavlidou, P. Szoleczky, D. Geroe, K. Yanagi, G. Toeroe, I. Lopez-Garcia, V. Myrianthopoulos, E. Mikros, J. R. Zatarain, C. Chao, A. Papapetropoulos, M. R. Hellmich, C. Szabo, Pharmacol. Res. 2016, 113, 18–37.
- 36M. Goyal, G. Tulsyan, D. D. Kanabar, T. Chavan, A. Muth, V. Gupta, J. Drug Deliv. Sci. Technol. 2023, 87, 104767.
- 37P. Ronnander, L. Simon, H. Spilgies, A. Koch, S. Scherr, Eur. J. Pharm. Sci. 2018, 114, 84–92.
- 38T. Chen, R. Huang, J. Liang, B. Zhou, X. Guo, X. Shen, B. Jiang, Chem. Eur. J. 2020, 26, 15159–15169.
- 39D. Yu, Z. Wei, X. Zhang, Y. Zeng, C. Wang, G. Chen, Z. Shen, F. Du, Adv. Funct. Mater. 2021, 31, 2008743.
- 40C. Wang, F. Cao, Y. Ruan, X. Jia, W. Zhen, X. Jiang, Angew. Chem. Int. Ed. 2019, 58, 9846–9850.
- 41L. Lai, H. Ji, H. Zhang, R. Liu, C. Zhou, W. Liu, Z. Ao, N. Li, C. Liu, G. Yao, B. Lai, Appl. Catal. B Environ. 2021, 282, 119559.
- 42W. H. Wanna, D. Janmanchi, N. Thiyagarajan, R. Ramu, Y. F. Tsai, C. W. Pao, S. S. F. Yu, New J. Chem. 2019, 43, 17819–17830.
- 43B. Zhang, L. Wang, Y. Zhang, Y. Ding, Y. Bi, Angew. Chem. Int. Ed. 2018, 57, 2248–2252.
- 44Y. Wu, L. Ma, J. Wu, M. Song, C. Wang, J. Lu, Adv. Mater. 2024, 36, 2311698.
- 45L. Wang, X. Li, S. Xiong, H. Lin, Y. Xu, Y. Jiao, J. Chen, J. Colloid Interface Sci. 2021, 600, 58–71.
- 46W. Fan, H. Li, F. Zhao, X. Xiao, Y. Huang, H. Ji, Y. Tong, Chem. Commun. 2016, 52, 5316–5319.
- 47F. Haghighat, M. Mokhtary, J. Inorg. Organomet. Polym. Mater. 2017, 27, 779–787.
- 48I. Benisti, R. Nandi, N. Amdursky, Y. Paz, Appl. Catal. B. Environ. 2020, 278, 119351.
- 49Y. K. Kho, W. Y. Teoh, A. Iwase, L. Mädler, A. Kudo, R. Amal, ACS Appl. Mater. Interfaces 2011, 3, 1997–2004.
- 50M. S. Refat, S. B. Bakare, T. Altalhi, R. F. Hassan, J. Mol. Liq. 2021, 328, 115493.
- 51Z. Pei, H. Lei, J. Wu, W. Tang, K. Wei, L. Wang, F. Gong, N. Yang, L. Liu, Y. Yang, L. Cheng, ACS Nano 2023, 17, 17105–17121.
- 52S. Kumar, S. Kumari, R. Karan, A. Kumar, R. K. Rawal, P. K. Gupta, Inorg. Chem. Commun. 2024, 161, 112014.
- 53A. Samuni, D. Meisel, G. Czapski, J. Chem. Soc. Dalton Trans. 1972, 12, 1273–1277.
- 54V. Vacque, B. Sombret, J. P. Huvenne, P. Legrand, S. Suc, Spectrochim. Acta A Mol. Biomol. Spectrosc. 1997, 53, 55–66.
- 55Y. Tu, Angew. Chem. Int. Ed. 2016, 55, 10210–10226.
- 56Q. Liu, G. Huang, H. He, Q. Xu, H. Li, J. Liu, X. Liu, L. Mao, S. Kirk, S. Su, D. Yin, Catal. Commun. 2020, 142, 106041.
- 57G. Liu, J. Zhu, H. Guo, A. Sun, P. Chen, L. Xi, W. Huang, X. Song, X. Dong, Angew. Chem. Int. Ed. 2019, 58, 18641–18646.
- 58Y. Dong, S. Dong, Z. Wang, L. Feng, Q. Sun, G. Chen, F. He, S. Liu, W. Li, P. Yang, ACS Appl. Mater. Interfaces 2020, 12, 52479–52491.
- 59N. Katsaros, E. Vrachnouastra, J. Konstantatos, J. Inorg. Biochem. 1982, 16, 227–235.
- 60Y. Huang, J. Ren, X. Qu, Chem. Rev. 2019, 119, 4357–4412.
- 61C. Xu, Z. Liu, L. Wu, J. Ren, X. Qu, Adv. Funct. Mater. 2014, 24, 1624–1630.
- 62L. Vilčiauskas, M. E. Tuckerman, G. Bester, S. J. Paddison, K. D. Kreuer, Nat. Chem. 2012, 4, 461–466.
- 63L. Wang, Y. Wang, Z. Li, T. Li, R. Zhang, J. Li, B. Liu, Z. Lv, W. Cai, S. Sun, W. Hu, Y. Lu, G. Zhu, Adv. Mater. 2023, 35, e2303535.
- 64X. Li, B. Lv, X. Zhang, X. Jin, K. Guo, D. Zhou, H. Bian, W. Zhang, U. P. Apfel, R. Cao, Angew. Chem. Int. Ed. 2022, 61, e202114310.
- 65A. Epp, T. Ramasarma, L. R. Wetter, J. Am. Chem. Soc. 1958, 80, 724–727.
- 66S. Li, L. Wu, X. Zhang, X. Jiang, Angew. Chem. Int. Ed. 2020, 59, 6627–6630.
- 67Z. Ren, S. Sun, R. Sun, G. Cui, L. Hong, B. Rao, A. Li, Z. Yu, Q. Kan, Z. Mao, Adv. Mater. 2020, 32, 1906024.
- 68M. V. Zamaraeva, R. Z. Sabirov, E. Maeno, Y. Ando-Akatsuka, S. V. Bessonova, Y. Okada, Cell Death Differ. 2005, 12, 1390–1397.
- 69M. R. Elliott, F. B. Chekeni, P. C. Trampont, E. R. Lazarowski, A. Kadl, S. F. Walk, D. Park, R. I. Woodson, M. Ostankovich, P. Sharma, J. J. Lysiak, T. K. Harden, N. Leitinger, K. S. Ravichandran, Nature 2009, 461, 282–286.
- 70A. Terman, B. Gustafsson, U. T. Brunk, Chem. Biol. Interact. 2006, 163, 29–37.
- 71H. Wang, D. Jiao, D. Feng, Q. Liu, Y. Huang, J. Hou, D. Ding, W. Zhang, Adv. Mater. 2024, 36, 2311733.
- 72Y. Chen, Z. Yang, S. Wang, Q. Ma, L. Li, X. Wu, Q. Guo, L. Tao, X. Shen, Adv. Healthcare Mater. 2023, 12, 2202150.
- 73C. Mao, B. Gan, Nat. Cell Biol. 2022, 24, 1186–1187.
- 74M. Yamamoto, T. W. Kensler, H. Motohashi, Physiol. Rev. 2018, 98, 1169–1203.
- 75S. Fu, Y. Li, L. Shen, Y. Chen, J. Lu, Y. Ran, Y. Zhao, H. Tang, L. Tan, Q. Lin, Y. Hao, Small 2024, 20, 2309537.