Ligand-Controlled Regiodivergent Carbosilylation of 1,3-Dienes via Nickel-Catalyzed Three-Component Coupling Reactions
This article relates to:
-
Li-Jun Xiao
- Volume 64Issue 25Angewandte Chemie International Edition
- First Published online: April 27, 2025
Shan Jiang
State Key Laboratory and Institute of Elemento-Organic Chemistry, College of Chemistry, Frontiers Science Center for New Organic Matter, Nankai University, Tianjin, 300071 China
Search for more papers by this authorTianze Zhang
State Key Laboratory and Institute of Elemento-Organic Chemistry, College of Chemistry, Frontiers Science Center for New Organic Matter, Nankai University, Tianjin, 300071 China
Search for more papers by this authorXiao-Yuan Luo
State Key Laboratory and Institute of Elemento-Organic Chemistry, College of Chemistry, Frontiers Science Center for New Organic Matter, Nankai University, Tianjin, 300071 China
Search for more papers by this authorShoucheng Dong
State Key Laboratory and Institute of Elemento-Organic Chemistry, College of Chemistry, Frontiers Science Center for New Organic Matter, Nankai University, Tianjin, 300071 China
Search for more papers by this authorJin-Tao Ma
State Key Laboratory and Institute of Elemento-Organic Chemistry, College of Chemistry, Frontiers Science Center for New Organic Matter, Nankai University, Tianjin, 300071 China
Search for more papers by this authorCorresponding Author
Li-Jun Xiao
State Key Laboratory and Institute of Elemento-Organic Chemistry, College of Chemistry, Frontiers Science Center for New Organic Matter, Nankai University, Tianjin, 300071 China
E-mail: [email protected]
Search for more papers by this authorShan Jiang
State Key Laboratory and Institute of Elemento-Organic Chemistry, College of Chemistry, Frontiers Science Center for New Organic Matter, Nankai University, Tianjin, 300071 China
Search for more papers by this authorTianze Zhang
State Key Laboratory and Institute of Elemento-Organic Chemistry, College of Chemistry, Frontiers Science Center for New Organic Matter, Nankai University, Tianjin, 300071 China
Search for more papers by this authorXiao-Yuan Luo
State Key Laboratory and Institute of Elemento-Organic Chemistry, College of Chemistry, Frontiers Science Center for New Organic Matter, Nankai University, Tianjin, 300071 China
Search for more papers by this authorShoucheng Dong
State Key Laboratory and Institute of Elemento-Organic Chemistry, College of Chemistry, Frontiers Science Center for New Organic Matter, Nankai University, Tianjin, 300071 China
Search for more papers by this authorJin-Tao Ma
State Key Laboratory and Institute of Elemento-Organic Chemistry, College of Chemistry, Frontiers Science Center for New Organic Matter, Nankai University, Tianjin, 300071 China
Search for more papers by this authorCorresponding Author
Li-Jun Xiao
State Key Laboratory and Institute of Elemento-Organic Chemistry, College of Chemistry, Frontiers Science Center for New Organic Matter, Nankai University, Tianjin, 300071 China
E-mail: [email protected]
Search for more papers by this authorGraphical Abstract
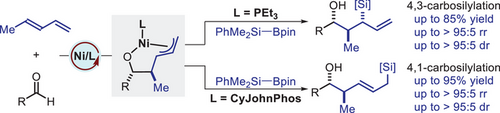
A regiodivergent nickel-catalyzed carbosilylation of 1,3-dienes using aldehydes and silylboranes has been developed, with ligand control influencing the reaction pathway. The employment of triethylphosphine promotes 4,3-addition selectivity, whereas using (2-biphenyl)dicyclohexylphosphine leads to 4,1-addition selectivity.
Abstract
The regiodivergent carbosilylation of 1,3-dienes presents a formidable challenge due to inherently complex selectivity control over multiple potential reaction pathways. Here, we report a ligand-controlled, regiodivergent carbosilylation of 1,3-dienes with aldehydes and silylboranes, achieving unprecedented site-selectivity using nickel catalysts with distinct phosphine ligands. The use of triethylphosphine promotes 4,3-addition selectivity, while employing (2-biphenyl)dicyclohexylphosphine facilitates 4,1-addition selectivity. This method displays excellent regio- and diastereoselectivity, as well as a broad substrate scope and substantial functional group tolerance. Mechanistic studies indicate that the ligand choice is crucial for directing the reaction pathway and stabilizing π-allyl-nickel intermediates. Our protocol provides a practical and efficient approach to synthesizing valuable functionalized allylsilanes, which are important in various synthetic applications.
Conflict of Interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the Supporting Information of this article.
Supporting Information
| Filename | Description |
|---|---|
| anie202504494-sup-0001-SuppMat.pdf16.4 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1A. M. Canfield, D. Rodina, S. M. Paradine, Angew. Chem. Int. Ed. 2024, 63, e202401550; Angew. Chem. 2024, 136, e202401550.
- 2G. J. P. Perry, T. Jia, D. J. Procter, ACS Catal. 2020, 10, 1485–1499.
- 3N. Herrmann, D. Vogelsang, A. Behr, T. Seidensticker, ChemCatChem 2018, 10, 5342–5365.
- 4X. Wu, L.-Z. Gong, Synthesis 2019, 51, 122–134.
- 5N. J. Adamson, S. J. Malcolmson, ACS Catal. 2020, 10, 1060–1076.
- 6A. Flaget, C. Zhang, C. Mazet, ACS Catal. 2022, 12, 15638–15647.
- 7G. Li, X. Huo, X. Jiang, W. Zhang, Chem. Soc. Rev. 2020, 49, 2060–2118.
- 8L. T. Kliman, S. N. Mlynarski, G. E. Ferris, J. P. Morken, Angew. Chem. Int. Ed. 2012, 51, 521–524; Angew. Chem. 2012, 124, 536–539.
- 9D.-F. Lu, C.-L. Zhu, J. D. Sears, H. Xu, J. Am. Chem. Soc. 2016, 138, 11360–11367.
- 10K. P. S. Cheung, D. Kurandina, T. Yata, V. Gevorgyan, J. Am. Chem. Soc. 2020, 142, 9932–9937.
- 11J. Chen, Y.-J. Liang, P.-Z. Wang, G.-Q. Li, B. Zhang, H. Qian, X.-D. Huan, W. Guan, W.-J. Xiao, J.-R. Chen, J. Am. Chem. Soc. 2021, 143, 13382–13392.
- 12H. Li, J. Long, Y. Li, W. Wang, H. Pang, G. Yin. Eur. J. Org. Chem. 2021, 2021, 1424–1428.
- 13L.-F. Fan, R. Liu, X.-Y. Ruan, P.-S. Wang, L.-Z. Gong, Nat. Synth. 2022, 1, 946–955.
- 14W.-S. Zhang, D.-W. Ji, Y. Li, X.-X. Zhang, C.-Y. Zhao, Y.-C. Hu, Q.-A. Chen, ACS Catal. 2022, 12, 2158–2165.
- 15Y. Cai, G. Gaurav, T. Ritter, Angew. Chem. Int. Ed. 2024, 63, e202311250; Angew. Chem. 2024, 136, e202311250.
- 16H. Du, W. Yuan, B. Zhao, Y. Shi, J. Am. Chem. Soc. 2007, 129, 11688–11689.
- 17F. Burg, T. Rovis, J. Am. Chem. Soc. 2021, 143, 17964–17969.
- 18Z.-L. Tao, A. Adili, H.-C. Shen, Z.-Y. Han, L.-Z. Gong, Angew. Chem. Int. Ed. 2016, 55, 4322–4326; Angew. Chem. 2016, 128, 4394–4398.
- 19X. Li, F. Meng, S. Torker, Y. Shi, A. H. Hoveyda, Angew. Chem. Int. Ed. 2016, 55, 9997–10002; Angew. Chem. 2016, 128, 10151–10156.
- 20A. Tortajada, O. Ninokata, R. Martin, J. Am. Chem. Soc. 2018, 140, 2050–2053.
- 21H.-M. Huang, M. Koy, E. Serrano, P. M. J. Pflüger, L. Schwarz, F. Glorius, Nat. Catal. 2020, 3, 393–400.
- 22K. Zhuang, G. C. Haug, Y. Wang, S. Yin, H. Sun, S. Huang, R. Trevino, K. Shen, Y. Sun, C. Huang, B. Qin, Y. Liu, M. Cheng, O. V. Larionov, S. Jin, J. Am. Chem. Soc. 2024, 146, 8508–8519.
- 23Y.-Y. Gui, X.-W. Chen, X.-Y. Mo, J.-P. Yue, R. Yuan, Y. Liu, L.-L. Liao, J.-H. Ye, D.-G. Yu, J. Am. Chem. Soc. 2024, 146, 2919–2927.
- 24C. Qi, H. Jiang, Chin. J. Org. Chem. 2024, 44, 1368.
- 25L. Jiang, P. Cao, M. Wang, B. Chen, B. Wang, J. Liao, Angew. Chem. Int. Ed. 2016, 55, 13854–13858; Angew. Chem. 2016, 128, 14058–14062.
- 26J.-J. Feng, M. Oestreich, Angew. Chem. Int. Ed. 2019, 58, 8211–8215; Angew. Chem. 2019, 131, 8295–8299.
- 27H. Wang, C.-F. Liu, T.-D. Tan, K. R. B. Khoo, M. J. Koh, ACS Catal. 2022, 12, 724–732.
- 28Y. Xiong, G. Zhang, J. Am. Chem. Soc. 2018, 140, 2735–2738.
- 29G. Feng, C. K. Ku, J. Zhao, Q. Wang, J. Am. Chem. Soc. 2022, 144, 20463–20471.
- 30J. Vaith, D. Rodina, G. C. Spaulding, S. M. Paradine, J. Am. Chem. Soc. 2022, 144, 6667–6673.
- 31B. Jiang, H.-T. Wang, Z.-C. Chen, W. Du, Y.-C. Chen, ACS Catal. 2024, 14, 628–636.
- 32S. Cai, Z. Zhao, G. Yang, H. Huang, Nat. Chem. 2024, 16, 1972–1981.
- 33H. Yao, W. Ji, Y. Feng, C. Qian, C. Yue, Y. Wang, S. Huang, M.-Y. Wang, X. Ma, Chin. Chem. Lett. 2025, 36, 110076.
- 34X. Ma, S. J. Malcolmson, J. Am. Chem. Soc. 2023, 145, 27680–27689.
- 35T. Jia, Q. He, R. E. Ruscoe, A. P. Pulis, D. J. Procter, Angew. Chem. Int. Ed. 2018, 57, 11305–11309; Angew. Chem. 2018, 130, 11475–11479.
- 36K. B. Smith, M. K. Brown, J. Am. Chem. Soc. 2017, 139, 7721–7724.
- 37S. R. Sardini, M. K. A. Brown, J. Am. Chem. Soc. 2017, 139, 9823–9826.
- 38C. E. Masse, J. S. Panek, Chem. Rev. 1995, 95, 1293–1316.
- 39E. Langkopf, D. Schinzer, Chem. Rev. 1995, 95, 1375–1408.
- 40I. Fleming, A. Barbero, D. Walter, Chem. Rev. 1997, 97, 2063–2192.
- 41L. Chabaud, P. James, Y. Landais, Eur. J. Org. Chem. 2004, 2004, 3173–3199.
- 42A. Rahimi, A. Schmidt, Synlett 2010, 2010, 1327-1330.
- 43C. Díez-Poza, A. Barbero, Eur. J. Org. Chem. 2017, 2017, 4651–4665.
- 44D. D. Roberts, M. G. McLaughlin, Adv. Synth. Catal. 2022, 364, 2307–2332.
- 45M. A. Brook, Silicon in Organic, Organometallic, and Polymer Chemistry. Wiley, New York 2000.
- 46Y. Obora, Y. Tsuji, T. Kawamura, J. Am. Chem. Soc. 1993, 115, 10414–10415.
- 47Y. Obora, Y. Tsuji, T. Kawamura, J. Am. Chem. Soc. 1995, 117, 9814–9821.
- 48N. Saito, A. Kobayashi, Y. Sato, Angew. Chem. Int. Ed. 2012, 51, 1228–1231; Angew. Chem. 2012, 124, 1254–1257.
- 49M. Suginome, H. Nakamura, T. Matsuda, Y. Ito, J. Am. Chem. Soc. 1998, 120, 4248–4249.
- 50M. Oestreich, E. Hartmann, M. Mewald, Chem. Rev. 2013, 113, 402–441.
- 51J.-J. Feng, W. Mao, L. Zhang, M. Oestreich, Chem. Soc. Rev. 2021, 50, 2010–2073.
- 52Y. Matsuda, Y. Tsuji, T. Fujihara, Chem. Commun. 2020, 56, 4648–4651.
- 53Q.-Q. Pan, L. Qi, X. Pang, X.-Z. Shu, Angew. Chem. Int. Ed. 2023, 62, e202215703; Angew. Chem. 2023, 135, e202215703.
- 54Y. Tani, T. Fujihara, J. Terao, Y. Tsuji, J. Am. Chem. Soc. 2014, 136, 17706–17709.
- 55T. Inagaki, S. Sakurai, M. Yamanaka, M. Tobisu, Angew. Chem. Int. Ed. 2022, 61, e202202387; Angew. Chem. 2022, 134, e202202387.
- 56J. Zhou, B. Jiang, Y. Fujihira, Z. Zhao, T. Imai, N. Shibata, Nat. Commun. 2021, 12, 3749.
- 57C. Ding, Y. Ren, Y. Yu, G. Yin, Nat. Commun. 2023, 14, 7670.
- 58Y. Sato, M. Takimoto, K. Hayashi, T. Katsuhara, K. Takagi, M. Mori, J. Am. Chem. Soc. 1994, 116, 9771–9772.
- 59M. Kimura, A. Ezoe, K. Shibata, Y. Tamaru, J. Am. Chem. Soc. 1998, 120, 4033–4034.
- 60H. Y. Cho, J. P. Morken, J. Am. Chem. Soc. 2008, 130, 16140–16141.
- 61H. Y. Cho, J. P. Morken, J. Am. Chem. Soc. 2010, 132, 7576–7577.
- 62T. Q. Davies, J. Y. Kim, A. Fürstner, J. Am. Chem. Soc. 2022, 144, 18817–18822.
- 63J. S. Marcum, S. J. Meek, J. Am. Chem. Soc. 2022, 144, 19231–19237.
- 64E. P. Jackson, H. A. Malik, G. J. Sormunen, R. D. Baxter, P. Liu, H. Wang, A.-R. Shareef, J. Montgomery, Acc. Chem. Res. 2015, 48, 1736–1745.
- 65E. A. Standley, S. Z. Tasker, K. L. Jensen, T. F. Jamison, Acc. Chem. Res. 2015, 48, 1503–1514.
- 66Y. Hoshimoto, M. Ohashi, S. Ogoshi, Acc. Chem. Res. 2015, 48, 1746–1755.
- 67For isolation of nickelacycles from oxidative cyclization of dienes and aldehydes with nickel(0), see: S. Ogoshi, K. Tonomori, M. Oka, H. Kurosawa, J. Am. Chem. Soc. 2006, 128, 7077–7086.
- 68B. Chen, Y. Zhang, R. Wu, D. Fang, X. Chen, S. Wang, Y. Zhao, P. Hu, K.-Q. Zhao, B.-Q. Wang, P. Cao, ACS Catal. 2019, 9, 11788–11793.
- 69Y.-Q. Li, G. Chen, S.-L. Shi, Org. Lett. 2021, 23, 2571–2577.
- 70S. Ma, F. Li, G. Zhang, L. Shi, X. Wang, ACS Catal. 2021, 11, 14848–14853.
- 71D. Ding, L.-F. Fan, Z.-Y. Han, P.-S. Wang, Org. Lett. 2023, 25, 210–214.
- 72T. Zhang, S. Jiang, M.-Y. Qian, Q.-L. Zhou, L.-J. Xiao, J. Am. Chem. Soc. 2024, 146, 3458–3470.
- 73K.-X. Zhang, M.-Y. Liu, B.-Y. Yao, Q.-L. Zhou, L.-J. Xiao, J. Am. Chem. Soc. 2024, 146, 22157–22165.
- 74J.-T. Ma, T. Zhang, B.-Y. Yao, L.-J. Xiao, Q.-L. Zhou, J. Am. Chem. Soc. 2023, 145, 19195–19201.
- 75Z.-H. Chen, L.-J. Gu, B. Wang, L.-J. Xiao, M. Ye, Q.-L. Zhou, J. Am. Chem. Soc. 2024, 146, 14915–14921.
- 76J.-T. Ma, L.-J. Xiao, Synlett 2025, 36, 1–7.
- 77M.-Y. Qian, K.-X. Zhang, L.-J. Xiao, Org. Chem. Front. 2024, 11, 4602–4623.
- 78J. Rohr, Angew. Chem. Int. Ed. 2000, 39, 2847–2849;
10.1002/1521-3773(20000818)39:16<2847::AID-ANIE2847>3.0.CO;2-0 CAS PubMed Web of Science® Google ScholarAngew. Chem. 2000, 112, 2967–2969.
- 79K.-S. Yeung, I. Paterson, Chem. Rev. 2005, 105, 4237–4313.
- 80A.-M. R. Dechert-Schmitt, D. C. Schmitt, X. Gao, T. Itoh, M. J. Krische, Nat. Prod. Rep. 2014, 31, 504.
- 81K. Wu, A. G. Doyle, Nat. Chem. 2017, 9, 779–784.
- 82S. H. Newman-Stonebraker, S. R. Smith, J. E. Borowski, E. Peters, T. Gensch, H. C. Johnson, M. S. Sigman, A. G. Doyle, Science 2021, 374, 301–308.
- 83The phosphine structures and descriptors from the Kraken Phosphine Library can be accessed through a web application (https://kraken.cs.toronto.edu/search). The Vbur (min) (minimum percent buried volume) values for specific ligands in the library are measured in cubic angstroms (Å3). To convert these values to %Vbur (min), divide by 179.59 Å3. This divisor represents the volume of a sphere with a radius of 3.5 Å.
- 84M.-M. Li, T. Zhang, L. Cheng, W.-G. Xiao, J.-T. Ma, L.-J. Xiao, Q.-L. Zhou, Nat. Commun. 2023, 14, 3326.
- 85Deposition Number(s) 2389070 (for 3a) contain(s) the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service.
- 86L. Nattmann, J. Cornella, Organometallics 2020, 39, 3295–3300.
- 87T. Matsumoto, Y. Kitano, F. Sato, Tetrahedron Lett. 1988, 29, 5685–5688.
- 88J. Liu, M. Chen, Chem. Sci. 2023, 14, 8103–8108.
- 89L. F. Peña, A. Barbero, Org. Lett. 2024, 26, 5202–5207.
- 90F. Peng, D. G. Hall, J. Am. Chem. Soc. 2007, 129, 3070–3071.
- 91Y. Gan, W. Xu, Y. Liu, Org. Lett. 2019, 21, 9652–9657.
- 92T. Moragas, J. Cornella, R. Martin, J. Am. Chem. Soc. 2014, 136, 17702–17705.
- 93P. Liu, P. McCarren, P. H.-Y. Cheong, T. F. Jamison, K. N. Houk, J. Am. Chem. Soc. 2010, 132, 2050–2057.
- 94For computational details, see the Supporting Information.