Synthesis of Chiral δ-Aminoboronic Esters by Enantioselective Hydrogenation of 1,2-Azaborines
Jiangpeng Liu
Department of Chemistry, Boston College, 2609 Beacon Street, Merkert Chemistry Center, Chestnut Hill, MA, 02467 USA
Search for more papers by this authorYuping Dai
E2S UPPA/CNRS, Institut des Sciences Analytiques et de Physico-Chimie pour l'Environnement et les Matériaux IPREM UMR 5254, Université de Pau et des Pays de l'Adour, Pau Cedex 09, 64053 France
Search for more papers by this authorDevon Robinson
Department of Chemistry, Boston College, 2609 Beacon Street, Merkert Chemistry Center, Chestnut Hill, MA, 02467 USA
Search for more papers by this authorBo Li
Department of Chemistry, Boston College, 2609 Beacon Street, Merkert Chemistry Center, Chestnut Hill, MA, 02467 USA
Search for more papers by this authorCorresponding Author
Karinne Miqueu
E2S UPPA/CNRS, Institut des Sciences Analytiques et de Physico-Chimie pour l'Environnement et les Matériaux IPREM UMR 5254, Université de Pau et des Pays de l'Adour, Pau Cedex 09, 64053 France
E-mail: [email protected]; [email protected]
Search for more papers by this authorCorresponding Author
Shih-Yuan Liu
Department of Chemistry, Boston College, 2609 Beacon Street, Merkert Chemistry Center, Chestnut Hill, MA, 02467 USA
E2S UPPA/CNRS, Institut des Sciences Analytiques et de Physico-Chimie pour l'Environnement et les Matériaux IPREM UMR 5254, Université de Pau et des Pays de l'Adour, Pau Cedex 09, 64053 France
E-mail: [email protected]; [email protected]
Search for more papers by this authorJiangpeng Liu
Department of Chemistry, Boston College, 2609 Beacon Street, Merkert Chemistry Center, Chestnut Hill, MA, 02467 USA
Search for more papers by this authorYuping Dai
E2S UPPA/CNRS, Institut des Sciences Analytiques et de Physico-Chimie pour l'Environnement et les Matériaux IPREM UMR 5254, Université de Pau et des Pays de l'Adour, Pau Cedex 09, 64053 France
Search for more papers by this authorDevon Robinson
Department of Chemistry, Boston College, 2609 Beacon Street, Merkert Chemistry Center, Chestnut Hill, MA, 02467 USA
Search for more papers by this authorBo Li
Department of Chemistry, Boston College, 2609 Beacon Street, Merkert Chemistry Center, Chestnut Hill, MA, 02467 USA
Search for more papers by this authorCorresponding Author
Karinne Miqueu
E2S UPPA/CNRS, Institut des Sciences Analytiques et de Physico-Chimie pour l'Environnement et les Matériaux IPREM UMR 5254, Université de Pau et des Pays de l'Adour, Pau Cedex 09, 64053 France
E-mail: [email protected]; [email protected]
Search for more papers by this authorCorresponding Author
Shih-Yuan Liu
Department of Chemistry, Boston College, 2609 Beacon Street, Merkert Chemistry Center, Chestnut Hill, MA, 02467 USA
E2S UPPA/CNRS, Institut des Sciences Analytiques et de Physico-Chimie pour l'Environnement et les Matériaux IPREM UMR 5254, Université de Pau et des Pays de l'Adour, Pau Cedex 09, 64053 France
E-mail: [email protected]; [email protected]
Search for more papers by this authorGraphical Abstract
Abstract
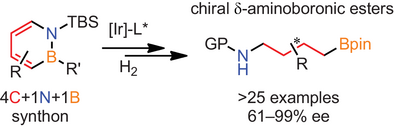
We describe herein an iridium-catalyzed highly diastereo- and enantioselective hydrogenation of 1,2-azaborines to access δ-aminoboronic esters of potential biological importance. This method represents the first enantioselective hydrogenation of a boron-containing heteroarene and features diverse substitution patterns and wide scope. The synthetic utility of our method was demonstrated by the synthesis of (−)-phenibut and the formal synthesis of (+)-3-PPP and fluvirucinine A1.
Conflict of Interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the Supporting Information of this article.
Supporting Information
| Filename | Description |
|---|---|
| anie202504419-sup-0001-SuppMat.pdf28.6 MB | Supporting Information |
| anie202504419-sup-0003-SuppMat.cif951 KB | Supporting Information |
| anie202504419-sup-0004-SuppMat.cif540.2 KB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1S. Touchet, F. Carreaux, B. Carboni, A. Bouillon, J.-L. Boucher, Chem. Soc. Rev. 2011, 40, 3895–3914.
- 2S. J. Baker, J. W. Tomsho, S. J. Benkovic, Chem. Soc. Rev. 2011, 40, 4279–4285.
- 3R. Smoum, A. Rubinstein, V. M. Dembitsky, M. Srebnik, Chem. Rev. 2012, 112, 4156–4220.
- 4A. Adamczyk-Woźniak, K. M. Borys, A. Sporzyński, Chem. Rev. 2015, 115, 5224–5247.
- 5D. B. Diaz, A. K. Yudin, Nat. Chem. 2017, 9, 731–742.
- 6G. F. S. Fernandes, W. A. Denny, J. L. Dos Santos, Eur. J. Med. Chem. 2019, 179, 791–804.
- 7S. Song, P. Gao, L. Sun, D. Kang, J. Kongsted, V. Poongavanam, P. Zhan, X. Liu, Acta Pharm. Sin. B 2021, 11, 3035–3059.
- 8B. C. Das, N. K. Nandwana, S. Das, V. Nandwana, M. A. Shareef, Y. Das, M. Saito, L. M. Weiss, F. Almaguel, N. S. Hosmane, T. Evans, Molecules 2022, 27, 2615.
- 9R. J. Grams, W. L. Santos, I. R. Scorei, A. Abad-García, C. A. Rosenblum, A. Bita, H. Cerecetto, C. Viñas, M. A. Soriano-Ursúa, Chem. Rev. 2024, 124, 2441–2511.
- 10J. Adams, Oncologist 2002, 7, 9–16.
- 11A. M. O'Farrell, A. van Vliet, K. Abou Farha, J. M. Cherrington, D. A. Campbell, X. Li, D. Hanway, J. Li, H.-P. Guler, Clin. Ther. 2007, 29, 1692–1705.
- 12A. S. Gorovoy, O. Gozhina, J.-S. Svendsen, G. V. Tetz, A. Domorad, V. V. Tetz, T. Lejon, J. Pept. Sci. 2013, 19, 613–618.
- 13A. Gzik, J. Chrzanowski, R. Blaszczyk, B. Borek, J. Olczak, A. Golebiowski, M. M. Grzybowski, P. Pomper, N. C. Güner-Chalimoniuk, K. Lisiecki, D. Kusmirek, WO 2022137156 A1, 2022.
- 14For an overview see: P. Andrés, G. Ballano, M. I. Calaza, C. Cativiela, Chem. Soc. Rev. 2016, 45, 2291–2307.
- 15A. Šterman, I. Sosič, S. Gobec, Z. Časar, Org. Chem. Front. 2019, 6, 2991–2998.
- 16W. Ming, H. S. Soor, X. Liu, A. Trofimova, A. K. Yudin, T. B. Marder, Chem. Soc. Rev. 2021, 50, 12151–12188.
- 17X. Li, D. G. Hall, J. Am. Chem. Soc. 2020, 142, 9063–9069.
- 18A. D. J. Calow, A. S. Batsanov, A. Pujol, C. Solé, E. Fernández, A. Whiting, Org. Lett. 2013, 15, 4810–4813.
- 19L. Jiang, P. Cao, M. Wang, B. Chen, B. Wang, J. Liao, Angew. Chem. Int. Ed. 2016, 55, 13854–13858.
- 20Y. Xi, J. F. Hartwig, J. Am. Chem. Soc. 2016, 138, 6703–6706.
- 21S. Zhang, J. del Pozo, F. Romiti, Y. Mu, S. Torker, A. H. Hoveyda, Science 2019, 364, 45–51.
- 22T. Wang, M. Wang, Y. Wang, M. Li, Y. Zheng, Q. Chen, Y. Zhao, Z. Shi, Chem 2023, 9, 130–142.
- 23J. M. Jego, B. Carboni, M. Vaultier, J. Organomet. Chem. 1992, 435, 1–8.
- 24S. Collet, P. Bauchat, R. Danion-Bougot, D. Danion, Tetrahedron Asymm 1998, 9, 2121–2131.
- 25P. V. Ramachandran, W. Mitsuhashi, D. Biswas, D. R. Nicponski, Tetrahedron Lett. 2013, 54, 4830–4833.
- 26A. Dicko, M. Montury, M. Baboulene, Synth. Commun. 1988, 18, 459–463.
- 27Y. Xi, J. F. Hartwig, J. Am. Chem. Soc. 2016, 138, 6703–6706.
- 28H. Zhao, Q. Gao, Y. Zhang, P. Zhang, S. Xu, Org. Lett. 2020, 22, 2861–2866.
- 29Y. Lee, H. Jang, A. H. Hoveyda, J. Am. Chem. Soc. 2009, 131, 18234–18235.
- 30S. Bera, R. Mao, X. Hu, Nat. Chem. 2021, 13, 270–277.
- 31C. Sun, Y. Li, G. Yin, Angew. Chem. Int. Ed. 2022, 61, e202209076.
- 32Z. Li, H. Shi, X. Chen, L. Peng, Y. Li, G. Yin, J. Am. Chem. Soc. 2023, 145, 13603–13614.
- 33Y.-S. Zhu, Y.-L. Guo, Y.-Y. Zhu, B. Su, J. Am. Chem. Soc. 2024, 146, 32283–32291.
- 34For select examples involving monocyclic 1,2-azaborines see: A. J. V. Marwitz, J. T. Jenkins, L. N. Zakharov, S.-Y. Liu, Angew. Chem. Int. Ed. 2010, 49, 7444–7447.
- 35T. Taniguchi, S. Yamaguchi, Organometallics 2010, 29, 5732–5735.
- 36A. J. V. Marwitz, A. N. Lamm, L. N. Zakharov, M. Vasiliu, D. A. Dixon, S.-Y. Liu, Chem. Sci. 2012, 3, 825–829.
- 37A. W. Baggett, F. Guo, B. Li, S.-Y. Liu, F. Jäkle, Angew. Chem. Int. Ed. 2015, 54, 11191–11195.
- 38H. Braunschweig, M. A. Celik, F. Hupp, I. Krummenacher, L. Mailänder, Angew. Chem. Int. Ed. 2015, 54, 6347–6351.
- 39W.-M. Wan, A. W. Baggett, F. Cheng, H. Lin, S.-Y. Liu, F. Jäkle, Chem. Commun. 2016, 52, 13616–13619.
- 40M. Chen, K. S. Unikela, R. Ramalakshmi, B. Li, C. Darrigan, A. Chrostowska, S.-Y. Liu, Angew. Chem. Int. Ed. 2021, 60, 1556–1560.
- 41D. H. Knack, J. L. Marshall, G. P. Harlow, A. Dudzik, M. Szaleniec, S.-Y. Liu, J. Heider, Angew. Chem. Int. Ed. 2013, 52, 2599–2601.
- 42H. Lee, M. Fischer, B. K. Shoichet, S.-Y. Liu, J. Am. Chem. Soc. 2016, 138, 12021–12024.
- 43P. Zhao, D. O. Nettleton, R. G. Karki, F. J. Zécri, S.-Y. Liu, ChemMedChem 2017, 12, 358–361.
- 44Y. Liu, S.-Y. Liu, Org. Biomol. Chem. 2019, 17, 7002–7006.
- 45R. J. Burford, B. Li, M. Vasiliu, D. A. Dixon, S.-Y. Liu, Angew. Chem. Int. Ed. 2015, 54, 7823–7827.
- 46K. Edel, X. Yang, J. S. A. Ishibashi, A. N. Lamm, C. Maichle-Mössmer, Z. X. Giustra, S.-Y. Liu, H. F. Bettinger, Angew. Chem. Int. Ed. 2018, 57, 5296–5300.
- 47Z. X. Giustra, X. Yang, M. Chen, H. F. Bettinger, S.-Y. Liu, Angew. Chem. Int. Ed. 2019, 58, 18918–18922.
- 48T. Ozaki, S. K. Bentley, N. Rybansky, B. Li, S.-Y. Liu, J. Am. Chem. Soc. 2024, 146, 24748–24753.
- 49T. Ozaki, S.-Y. Liu, Chem. - Eur. J. 2024, 30, e202402544.
- 50For an overview, see: C. R. McConnell, S.-Y. Liu, Chem. Soc. Rev. 2019, 48, 3436–3453.
- 51A. W. Baggett, M. Vasiliu, B. Li, D. A. Dixon, S.-Y. Liu, J. Am. Chem. Soc. 2015, 137, 5536–5541.
- 52A. N. Brown, B. Li, S.-Y. Liu, J. Am. Chem. Soc. 2015, 137, 8932–8935.
- 53C. R. McConnell, F. Haeffner, A. W. Baggett, S.-Y. Liu, J. Am. Chem. Soc. 2019, 141, 9072–9078.
- 54For an overview see: D.-S. Wang, Q.-A. Chen, S.-M. Lu, Y.-G. Zhou, Chem. Rev. 2012, 112, 2557–2590.
- 55Z. X. Giustra, J. S. A. Ishibashi, S.-Y. Liu, Coord. Chem. Rev. 2016, 314, 134–181.
- 56M. P. Wiesenfeldt, Z. Nairoukh, T. Dalton, F. Glorius, Angew. Chem. Int. Ed. 2019, 58, 10460–10476.
- 57A. N. Kim, B. M. Stoltz, ACS Catal. 2020, 10, 13834–13851.
- 58For selected examples see: R. Kuwano, K. Sato, T. Kurokawa, D. Karube, Y. Ito, J. Am. Chem. Soc. 2000, 122, 7614–7615.
- 59R. Kuwano, K. Kaneda, T. Ito, K. Sato, T. Kurokawa, Y. Ito, Org. Lett. 2004, 6, 2213–2215.
- 60R. Kuwano, M. Kashiwabara, Org. Lett. 2006, 8, 2653–2655.
- 61A. Baeza, A. Pfaltz, Chem. - Eur. J. 2010, 16, 2036–2039.
- 62D.-S. Wang, Q.-A. Chen, W. Li, C.-B. Yu, Y.-G. Zhou, X. Zhang, J. Am. Chem. Soc. 2010, 132, 8909–8911.
- 63A. M. Maj, I. Suisse, C. Méliet, F. Agbossou-Niedercorn, Tetrahedron Asymm 2010, 21, 2010–2014.
- 64Y. Duan, L. Li, M.-W. Chen, C.-B. Yu, H.-J. Fan, Y.-G. Zhou, J. Am. Chem. Soc. 2014, 136, 7688–7700.
- 65T. Touge, T. Arai, J. Am. Chem. Soc. 2016, 138, 11299–11305.
- 66Y. Ge, Z. Wang, Z. Han, K. Ding, Chem. - Eur. J. 2020, 26, 15482–15486.
- 67F. Zhang, H. S. Sasmal, C. G. Daniliuc, F. Glorius, J. Am. Chem. Soc. 2023, 145, 15695–15701.
- 68R. Kuwano, M. Kashiwabara, M. Ohsumi, H. Kusano, J. Am. Chem. Soc. 2008, 130, 808–809.
- 69D.-S. Wang, Z.-S. Ye, Q.-A. Chen, Y.-G. Zhou, C.-B. Yu, H.-J. Fan, Y. Duan, J. Am. Chem. Soc. 2011, 133, 8866–8869.
- 70K. Tian, G. Liu, X.-Q. Dong, Chin. Chem. Lett. 2022, 33, 5092–5095.
- 71I. Lapin, CNS Drug Rev. 2001, 7, 471–481.
- 72N. Langlois, N. Dahuron, H.-S. Wang, Tetrahedron 1996, 52, 15117–15126.
- 73For an overview, see: C. Sandford, V. K. Aggarwal, Chem. Commun. 2017, 53, 5481–5494.
- 74M. Zhao, J. Li, E. Mano, Z. Song, D. M. Tschaen, E. J. J. Grabowski, P. J. Reider, J. Org. Chem. 1999, 64, 2564–2566.
- 75K. Mathew Sadhu, D. S. Matteson, Organometallics 1985, 4, 1687–1689.
- 76D. J. A. Schedler, A. G. Godfrey, B. Ganem, Tetrahedron Lett. 1993, 34, 5035–5038.
- 77S. B. Hellewell, W. D. Bowen, Brain Res. 1990, 527, 244–253.
- 78S. B. Hellewell, A. Bruce, G. Feinstein, J. Orringer, W. Williams, W. D. Bowen, Eur. J. Pharmacol.: Mol. Pharmacol. Sect. 1994, 268, 9–18.
- 79L. Zhou, D. W. Tay, J. Chen, G. Y. C. Leung, Y.-Y. Yeung, Chem. Commun. 2013, 49, 4412–4414.
- 80S.-J. Zhang, W.-W. Sun, Q.-Y. Yu, P. Cao, X.-P. Dong, B. Wu, Tetrahedron Lett. 2017, 58, 606–609.
- 81R. P. Sonawane, V. Jheengut, C. Rabalakos, R. Larouche-Gauthier, H. K. Scott, V. K. Aggarwal, Angew. Chem. Int. Ed. 2011, 50, 3760–3763.
- 82N. Naruse, O. Tenmyo, K. Kawano, K. Tomita, N. Ohgusa, T. Miyaki, M. Konishi, T. Oki, J. Antibiot. 1991, 44, 733–740.
- 83B. Liang, E. Negishi, Org. Lett. 2008, 10, 193–195.
- 84T. L. Church, T. Rasmussen, P. G. Andersson, Organometallics 2010, 29, 6769–6781.
- 85Y. Ge, Z. Wang, Z. Han, K. Ding, Chem. - Eur. J. 2020, 26, 15482–15486.