Enhanced Luminance of Pentaazaphenalene-Based Delayed Fluorescence Emitters by Breaking Forbidden Transition
Graphical Abstract
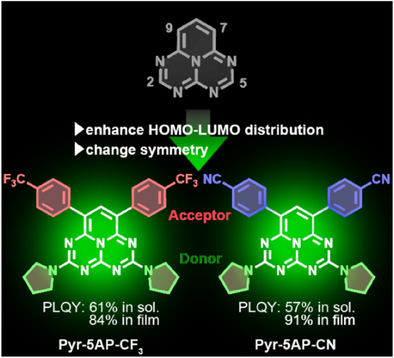
The newly developed 1,3,4,6,9b-pentaazaphenalene (5AP) based emitters with donor and acceptor units have succeeded in converting nonemissive 5AP to highly luminescent emitters by breaking the forbidden transition. These emitters exhibited photoluminescence quantum yields exceeding 80% and 90%; both are the highest among the 5AP derivatives reported to date.
Abstract
1,3,4,6,9b-pentaazaphenalene (5AP) derivatives are of growing interest because of their potential for exhibiting thermally activated delayed fluorescence and inverted singlet–triplet excited state properties. However, a major challenge has been the nonemissive nature of 5AP. This study reports a donor-5AP-acceptor-type molecular design for converting nonemissive 5AP into highly emissive molecules. The newly designed molecules, 2,5-di(1-pyrrolidino)-7,9-bis(4-(trifluoromethyl)phenyl)-1,3,4,6,9b-pentaazaphenalene (Pyr-5AP-CF3) and 2,5-di(1-pyrrolidino)-7,9-bis(4-benzonitrile)-1,3,4,6,9b-pentaazaphenalene (Pyr-5AP-CN), exhibited delayed fluorescence and achieved high photoluminescence quantum yields of 83.5% and 90.6%, respectively, in solid films. These values dramatically exceed those of previously reported 5AP derivatives with only 8% or less. Furthermore, Pyr-5AP-CF3 and Pyr-5AP-CN exhibited the fastest radiative decays and the narrowest emission spectra among all the 5AP based materials reported to date. This study provides a promising solution to the nonemissive nature of 5AP, leading to the development of a class of highly luminescent materials for future organic light-emitting diodes.
Conflict of Interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.