High-Performance Cu6Sn5 Alloy Electrocatalysts for Formaldehyde Oxidative Dehydrogenation and Bipolar Hydrogen Production
Graphical Abstract
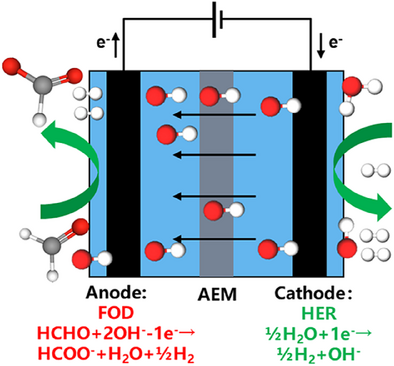
The Cu₆Sn₅ alloy was developed as a high-performance, noble-metal-free electrocatalyst for the anodic formaldehyde oxidative dehydrogenation (FOD) reaction. When paired with the cathodic hydrogen evolution reaction (HER), it enables a formaldehyde-assisted water electrolyzer, facilitating energy-efficient bipolar hydrogen production and the generation of value-added formate.
Abstract
Aldehyde-assisted water electrolysis offers an attractive pathway for energy-saving bipolar hydrogen production with combined faradaic efficiency (FE) of 200% while converting formaldehyde into value-added formate. Herein we report the design and synthesis of noble metal-free Cu6Sn5 alloy as a highly effective electrocatalyst for formaldehyde electro-oxidative dehydrogenation, demonstrating a geometric current density of 915 ± 46 mA cm−2 at 0.4 V versus reversible hydrogen electrode, outperforming many noble metal electrocatalysts reported previously. The formaldehyde-assisted water electrolyzer delivers 100 mA cm−2 at a low cell voltage of 0.124 V, and a current density of 486 ± 20 mA cm−2 at a cell voltage of 0.6 V without any iR compensation and exhibits nearly 200% faradaic efficiency for bipolar hydrogen production at 100 mA cm−2 in 88 h long-term operation. Density functional theory calculations further confirm the notably lowered barriers for dehydrogenation and Tafel steps on the Cu₆Sn₅ surface compared to Cu, underscoring its potential as a highly active catalyst.
Conflict of Interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.