N-(Acyldithio)Saccharin: Design, Synthesis and Applications in Catalytic Enantioselective Disulfuration/Amination of Alkenes
Yu-Xuan Huo
State Key Laboratory of Synergistic Chem-Bio Synthesis, School of Chemistry and Chemical Engineering, Shanghai Key Laboratory for Molecular Engineering of Chiral Drugs, Shanghai Jiao Tong University, Shanghai, 200240 P.R. China
Search for more papers by this authorRen-Fei Cao
State Key Laboratory of Synergistic Chem-Bio Synthesis, School of Chemistry and Chemical Engineering, Shanghai Key Laboratory for Molecular Engineering of Chiral Drugs, Shanghai Jiao Tong University, Shanghai, 200240 P.R. China
Search for more papers by this authorJie Huang
State Key Laboratory of Synergistic Chem-Bio Synthesis, School of Chemistry and Chemical Engineering, Shanghai Key Laboratory for Molecular Engineering of Chiral Drugs, Shanghai Jiao Tong University, Shanghai, 200240 P.R. China
Search for more papers by this authorZe-Long Li
State Key Laboratory of Synergistic Chem-Bio Synthesis, School of Chemistry and Chemical Engineering, Shanghai Key Laboratory for Molecular Engineering of Chiral Drugs, Shanghai Jiao Tong University, Shanghai, 200240 P.R. China
Search for more papers by this authorZheng-Wei Wei
State Key Laboratory of Synergistic Chem-Bio Synthesis, School of Chemistry and Chemical Engineering, Shanghai Key Laboratory for Molecular Engineering of Chiral Drugs, Shanghai Jiao Tong University, Shanghai, 200240 P.R. China
Search for more papers by this authorDeng Zhu
State Key Laboratory of Synergistic Chem-Bio Synthesis, School of Chemistry and Chemical Engineering, Shanghai Key Laboratory for Molecular Engineering of Chiral Drugs, Shanghai Jiao Tong University, Shanghai, 200240 P.R. China
Search for more papers by this authorCorresponding Author
Zhi-Min Chen
State Key Laboratory of Synergistic Chem-Bio Synthesis, School of Chemistry and Chemical Engineering, Shanghai Key Laboratory for Molecular Engineering of Chiral Drugs, Shanghai Jiao Tong University, Shanghai, 200240 P.R. China
E-mail: [email protected]
Search for more papers by this authorYu-Xuan Huo
State Key Laboratory of Synergistic Chem-Bio Synthesis, School of Chemistry and Chemical Engineering, Shanghai Key Laboratory for Molecular Engineering of Chiral Drugs, Shanghai Jiao Tong University, Shanghai, 200240 P.R. China
Search for more papers by this authorRen-Fei Cao
State Key Laboratory of Synergistic Chem-Bio Synthesis, School of Chemistry and Chemical Engineering, Shanghai Key Laboratory for Molecular Engineering of Chiral Drugs, Shanghai Jiao Tong University, Shanghai, 200240 P.R. China
Search for more papers by this authorJie Huang
State Key Laboratory of Synergistic Chem-Bio Synthesis, School of Chemistry and Chemical Engineering, Shanghai Key Laboratory for Molecular Engineering of Chiral Drugs, Shanghai Jiao Tong University, Shanghai, 200240 P.R. China
Search for more papers by this authorZe-Long Li
State Key Laboratory of Synergistic Chem-Bio Synthesis, School of Chemistry and Chemical Engineering, Shanghai Key Laboratory for Molecular Engineering of Chiral Drugs, Shanghai Jiao Tong University, Shanghai, 200240 P.R. China
Search for more papers by this authorZheng-Wei Wei
State Key Laboratory of Synergistic Chem-Bio Synthesis, School of Chemistry and Chemical Engineering, Shanghai Key Laboratory for Molecular Engineering of Chiral Drugs, Shanghai Jiao Tong University, Shanghai, 200240 P.R. China
Search for more papers by this authorDeng Zhu
State Key Laboratory of Synergistic Chem-Bio Synthesis, School of Chemistry and Chemical Engineering, Shanghai Key Laboratory for Molecular Engineering of Chiral Drugs, Shanghai Jiao Tong University, Shanghai, 200240 P.R. China
Search for more papers by this authorCorresponding Author
Zhi-Min Chen
State Key Laboratory of Synergistic Chem-Bio Synthesis, School of Chemistry and Chemical Engineering, Shanghai Key Laboratory for Molecular Engineering of Chiral Drugs, Shanghai Jiao Tong University, Shanghai, 200240 P.R. China
E-mail: [email protected]
Search for more papers by this authorGraphical Abstract
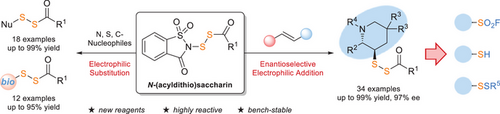
N-(Acyldithio)saccharins are a novel class of highly electrophilic disulfurating reagents. Beyond enabling the disulfuration of diverse N-, S-, and Cnucleophiles, the reagents facilitate the catalytic enantioselective disulfuration/amination of unactivated alkenes. The resulting α-chiral disulfide products can be readily converted into various chiral sulfur-containing compounds.
Abstract
We have designed and successfully synthesized N-(acyldithio)saccharin, which is a highly electrophilic, bench-stable, and user-friendly disulfurating reagent. This reagent can undergo reactions with diverse N-, S-, and C-nucleophiles at room temperature. In most cases, no additional catalyst is required, and the desired disulfides were readily obtained in moderate to excellent yields. With this reagent, late-stage disulfuration of pharmaceuticals and biomolecules was readily accomplished. For the first time, catalytic enantioselective disulfuration/amination of unactivated alkenes was achieved using this reagent. A series of chiral disulfides were obtained with high enantioselectivities and yields. The chiral disulfide products can be readily further transformed into chiral sulfonyl fluoride, chiral thiol, and structurally diverse disulfide products. Furthermore, we have evaluated the electrophilic reactivity of a series of disulfurating reagents based on density functional theory calculations, verifying the high reactivity of N-(acyldithio)saccharin both experimentally and theoretically.
Conflict of Interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.
Supporting Information
| Filename | Description |
|---|---|
| anie202503815-sup-0001-SuppMatS1.pdf18.3 MB | Supporting Information S1 |
| anie202503815-sup-0002-SuppMatS2.zip1.7 MB | Supporting Information S2 |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1A. J. Wommack, J. J. Ziarek, J. Tomaras, H. R. Chileveru, Y. Zhang, G. Wagner, E. M. Nolan, J. Am. Chem. Soc. 2014, 136, 13494–13497.
- 2S. Sparapani, C. Millet-Boureima, J. Oliver, K. Mu, P. Hadavi, T. Kalostian, N. Ali, C. M. Avelar, M. Bardies, B. Barrow, M. Benedikt, G. Biancardi, R. Bindra, L. Bui, Z. Chihab, A. Cossitt, J. Costa, T. Daigneault, J. Dault, I. Davidson, J. Dias, E. Dufour, S. El-Khoury, N. Farhangdoost, A. Forget, A. Fox, M. Gebrael, M. C. Gentile, O. Geraci, A. Gnanapragasam, et al., Biomedicines 2021, 9, 89.
- 3D. Fass, C. Thorpe, Chem. Rev. 2018, 118, 1169–1198.
- 4C. S. Sevier, C. A. Kaiser, Nat. Rev. Mol. Cell Biol. 2002, 3, 836–847.
- 5J. Alegre-Cebollada, P. Kosuri, J. A. Rivas-Pardo, J. M. Fernández, Nat. Chem. 2011, 3, 882–887.
- 6S. Lu, S.-B. Fan, B. Yang, Y.-X. Li, J.-M. Meng, L. Wu, P. Li, K. Zhang, M.-J. Zhang, Y. Fu, J. Luo, R.-X. Sun, S.-M. He, M.-Q. Dong, Nat. Methods 2015, 12, 329–331.
- 7M. Wensien, F. R. von Pappenheim, L.-M. Funk, P. Kloskowski, U. Curth, U. Diederichsen, J. Uranga, J. Ye, P. Fang, K.-T. Pan, H. Urlaub, R. A. Mata, V. Sautner, K. Tittmann, Nature 2021, 593, 460–464.
- 8M. Góngora-Benítez, J. Tulla-Puche, F. Albericio, Chem. Rev. 2014, 114, 901–926.
- 9Y. Liao, M. Wang, X. Jiang, Curr. Opin. Chem. Biol. 2023, 75, 102336.
- 10X. Ji, A. L. Nielsen, C. Heinis, Angew. Chem. Int. Ed. 2024, 63, e202308251.
- 11V. M. Dirsch, D. S. M. Antlsperger, H. Hentze, A. M. Vollmar, Leukemia 2002, 16, 74–83.
- 12B. Salehi, Y. Berkay Yılmaz, G. Antika, T. Boyunegmez Tumer, M. Fawzi Mahomoodally, D. Lobine, M. Akram, M. Riaz, E. Capanoglu, F. Sharopov, N. Martins, W. C. Cho, J. Sharifi-Rad, Biomolecules 2019, 9, 356.
- 13Z. Skrott, M. Mistrik, K. K. Andersen, S. Friis, D. Majera, J. Gursky, T. Ozdian, J. Bartkova, Z. Turi, P. Moudry, M. Kraus, M. Michalova, J. Vaclavkova, P. Dzubak, I. Vrobel, P. Pouckova, J. Sedlacek, A. Miklovicova, A. Kutt, J. Li, J. Mattova, C. Driessen, Q. P. Dou, J. Olsen, M. Hajduch, B. Cvek, R. J. Deshaies, J. Bartek, Nature 2017, 552, 194–199.
- 14S. Santra, C. Kaittanis, O. J. Santiesteban, J. M. Perez, J. Am. Chem. Soc. 2011, 133, 16680–16688.
- 15J. Zhao, X. Li, T. Ma, B. Chang, B. Zhang, J. Fang, Med. Res. Rev. 2024, 44, 1013–1054.
- 16R. V. J. Chari, M. L. Miller, W. C. Widdison, Angew. Chem. Int. Ed. 2014, 53, 3796–3827.
- 17A. Beck, L. Goetsch, C. Dumontet, N. Corvaïa, Nat. Rev. Drug Discovery 2017, 16, 315–337.
- 18N. Krall, F. Pretto, W. Decurtins, G. J. L. Bernardes, C. T. Supuran, D. Neri, Angew. Chem. Int. Ed. 2014, 53, 4231–4235.
- 19S. Ulrich, Acc. Chem. Res. 2019, 52, 510–519.
- 20W. Ma, X. Wang, D. Zhang, X. Mu, Int. J. Nanomed. 2024, 19, 7547–7566.
- 21W. Xiong, H. Lu, Sci. China Chem. 2023, 66, 2062–2069.
- 22Q. Zhang, Y.-X. Deng, H.-X. Luo, C.-Y. Shi, G. M. Geise, B. L. Feringa, H. Tian, D.-H. Qu, J. Am. Chem. Soc. 2019, 141, 12804–12814.
- 23Q. Zhang, Y. Deng, C.-Y. Shi, B. L. Feringa, H. Tian, D.-H. Qu, Matter 2021, 4, 1352–1364.
- 24B.-S. Wang, Q. Zhang, Z.-Q. Wang, C.-Y. Shi, X.-Q. Gong, H. Tian, D.-H. Qu, Angew. Chem. Int. Ed. 2023, 62, e202215329.
- 25M. Wang, X. Jiang, Top. Curr. Chem. 2018, 376, 14.
- 26C. L. Ong, S. Titinchi, J. C. Juan, N. G. Khaligh, Helv. Chim. Acta 2021, 104, e2100053.
- 27T. Mukaiyama, K. Takahashi, Tetrahedron Lett. 1968, 9, 5907–5908.
10.1016/S0040-4039(00)75437-2 Google Scholar
- 28P. Huang, P. Wang, S. Tang, Z. Fu, A. Lei, Angew. Chem. Int. Ed. 2018, 57, 8115–8119.
- 29H. Li, M. Peng, J. Li, L. Wang, H. Do, K. Ni, M. Wang, Z. Yuan, T. Zhao, X. Zhang, X. Zhang, Z. Hu, F. Ren, J. An, Nat. Commun. 2024, 15, 8325.
- 30D. N. Harpp, D. K. Ash, T. G. Back, J. G. Gleason, B. A. Orwig, W. F. VanHorn, J. P. Snyder, Tetrahedron Lett. 1970, 11, 3551–3554.
10.1016/S0040-4039(01)98525-9 Google Scholar
- 31D. N. Harpp, T. G. Back, J. Org. Chem. 1971, 36, 3828–3829.
- 32W.-C. Gao, K. Feng, J. Tian, J. Zhang, H.-H. Chang, X. Jiang, Chin. Chem. Lett. 2023, 34, 107587.
- 33Q. Yu, L. Bai, X. Jiang, Angew. Chem. Int. Ed. 2023, 62, e202314379.
- 34T. Yuan, X.-Y. Chen, T. Ji, H. Yue, K. Murugesan, M. Rueping, Chem. Sci. 2024, 15, 15474–15479.
- 35M. Arisawa, M. Yamaguchi, J. Am. Chem. Soc. 2003, 125, 6624–6625.
- 36X. Xiao, M. Feng, X. Jiang, Angew. Chem. Int. Ed. 2016, 55, 14121–14125.
- 37J. Zou, J. Chen, T. Shi, Y. Hou, F. Cao, Y. Wang, X. Wang, Z. Jia, Q. Zhao, Z. Wang, ACS Catal. 2019, 9, 11426–11430.
- 38F. Wang, Y. Chen, W. Rao, L. Ackermann, S.-Y. Wang, Nat. Commun. 2022, 13, 2588.
- 39X. Chen, W. Shao, G.-J. Deng, ACS Catal. 2024, 14, 6451–6461.
- 40X. Wang, W. Li, H. Wei, B. Wang, X. He, Y. Jiang, M. Nie, H. Yan, Z. Hu, Q. Gao, S. Qu, Angew. Chem. Int. Ed. 2025, e202424209.
- 41Q. Tian, Y. Li, Angew. Chem. Int. Ed. 2023, 62, e202302861.
- 42Q.-R. Dong, Y.-S. Wang, J. Zhang, H.-H. Chang, J. Tian, W.-C. Gao, ACS Catal. 2024, 14, 18237–18246.
- 43Z. Wu, D. A. Pratt, Nat. Rev. Chem. 2023, 7, 573–589.
- 44Z. Wu, D. A. Pratt, J. Am. Chem. Soc. 2020, 142, 10284–10290.
- 45Z. Wu, D. A. Pratt, Angew. Chem. Int. Ed. 2021, 60, 15598–15605.
- 46J. Zhang, A. Studer, Nat. Commun. 2022, 13, 3886.
- 47X. Ren, Q. Ke, Y. Zhou, J. Jiao, G. Li, S. Cao, X. Wang, Q. Gao, X. Wang, Angew. Chem. Int. Ed. 2023, 62, e202302199.
- 48H.-J. Huang, Z. Wu, D. A. Pratt, ACS Catal. 2023, 13, 13912–13919.
- 49L. G. S. Brooker, R. Child, S. Smiles, J. Chem. Soc. 1927, 1384–1388.
10.1039/JR9270001384 Google Scholar
- 50W. Wang, Y. Lin, Y. Ma, C.-H. Tung, Z. Xu, Org. Lett. 2018, 20, 3829–3832.
- 51Z. Zhang, T. Lao, L. Deng, C. Zhang, J. Liu, M. Fu, Z. Su, Y. Yu, H. Cao, Org. Lett. 2022, 24, 7222–7226.
- 52Y. Yu, J. Chen, M. Huang, Y. Jiang, X. Zhou, J. Wang, J. Li, H. Cao, J. Org. Chem. 2024, 89, 3590–3596.
- 53D. N. Harpp, D. K. Ash, Intl. J. Sulfur Chem. 1971, 1, 57–59.
- 54D. N. Harpp, T. G. Back, Tetrahedron Lett. 1972, 13, 1481–1484.
10.1016/S0040-4039(01)84662-1 Google Scholar
- 55D. N. Harpp, K. Steliou, T. H. Chan, J. Am. Chem. Soc. 1978, 100, 1222–1228.
- 56W.-C. Gao, J. Tian, Y.-Z. Shang, X. Jiang, Chem. Sci. 2020, 11, 3903–3908.
- 57W.-C. Gao, J. Liu, X. Jiang, Org. Chem. Front. 2021, 8, 1275–1279.
- 58D. Wang, W. Li, K. Shi, Y. Pan, J. Org. Chem. 2023, 88, 2550–2556.
- 59H. Asanuma, K. Kanemoto, Org. Lett. 2024, 26, 438–443.
- 60X. Xiao, J. Xue, X. Jiang, Nat. Commun. 2018, 9, 2191.
- 61Q. Zhang, Y. Li, L. Zhang, S. Luo, Angew. Chem. Int. Ed. 2021, 60, 10971–10976.
- 62S.-S. Zhang, J. Xue, Q. Gu, X. Jiang, S.-L. You, Org. Biomol. Chem. 2021, 19, 8761–8771.
- 63K. Kanemoto, K. Furuhashi, Y. Morita, T. Komatsu, S.-i. Fukuzawa, Org. Lett. 2021, 23, 1582–1587.
- 64H. Asanuma, K. Kanemoto, T. Watanabe, S.-i. Fukuzawa, Angew. Chem. Int. Ed. 2023, 62, e202219156.
- 65J. Xue, X. Jiang, Nat. Commun. 2020, 11, 4170.
- 66Q. Yu, X. Zhang, X. Jiang, Angew. Chem. Int. Ed. 2024, 63, e202408158.
- 67H. Böhme, M. Clement, Justus Liebigs Ann. Chem. 1952, 576, 61–69.
- 68H. Böhme, G. V. Ham, Justus Liebigs Ann. Chem. 1958, 617, 62–70.
- 69Y. Tian, X.-T. Li, J.-R. Liu, J. Cheng, A. Gao, N.-Y. Yang, Z. Li, K.-X. Guo, W. Zhang, H.-T. Wen, Z.-L. Li, Q.-S. Gu, X. Hong, X.-Y. Liu, Nat. Chem. 2024, 16, 466–475.
- 70C. Palomo, M. Oiarbide, F. Dias, R. López, A. Linden, Angew. Chem. Int. Ed. 2004, 43, 3307–3310.
- 71K. L. Kimmel, M. T. Robak, J. A. Ellman, J. Am. Chem. Soc. 2009, 131, 8754–8755.
- 72A. Peschiulli, B. Procuranti, C. J. O' Connor, S. J. Connon, Nat. Chem. 2010, 2, 380–384.
- 73S. Diosdado, J. Etxabe, J. Izquierdo, A. Landa, A. Mielgo, I. Olaizola, R. López, C. Palomo, Angew. Chem. Int. Ed. 2013, 52, 11846–11851.
- 74M. R. Monaco, S. Prévost, B. List, J. Am. Chem. Soc. 2014, 136, 16982–16985.
- 75N. Dong, Z.-P. Zhang, X.-S. Xue, X. Li, J.-P. Cheng, Angew. Chem. Int. Ed. 2016, 55, 1460–1464.
- 76M. Pickl, A. Swoboda, E. Romero, C. K. Winkler, C. Binda, A. Mattevi, K. Faber, M. W. Fraaije, Angew. Chem. Int. Ed. 2018, 57, 2864–2868.
- 77M. Li, J. Guo, X.-S. Xue, J.-P. Cheng, Org. Lett. 2016, 18, 264–267.
- 78F. Neese, WIREs Comput. Mol. Sci. 2012, 2, 73–78.
- 79F. Neese, F. Wennmohs, U. Becker, C. Riplinger, J. Chem. Phys. 2020, 152, 224108.
- 80F. Neese, WIREs Comput. Mol. Sci. 2022, 12, e1606.
- 81T. Lu, Q. Chen, Comput. Theor. Chem. 2021, 1200, 113249.
- 82S. E. Denmark, S. Rossi, M. P. Webster, H. Wang, J. Am. Chem. Soc. 2014, 136, 13016–13028.
- 83Y.-Y. Xie, Z.-M. Chen, H.-Y. Luo, H. Shao, Y.-Q. Tu, X. Bao, R.-F. Cao, S.-Y. Zhang, J.-M. Tian, Angew. Chem. Int. Ed. 2019, 58, 12491–12496.
- 84H.-Y. Luo, J.-W. Dong, Y.-Y. Xie, X.-F. Song, D. Zhu, T. Ding, Y. Liu, Z.-M. Chen, Chem. - Eur. J. 2019, 25, 15411–15418.
- 85H.-Y. Luo, Z.-H. Li, D. Zhu, Q. Yang, R.-F. Cao, T.-M. Ding, Z.-M. Chen, J. Am. Chem. Soc. 2022, 144, 2943–2952.
- 86D. Zhu, L. Yu, H.-Y. Luo, X.-S. Xue, Z.-M. Chen, Angew. Chem. Int. Ed. 2022, 61, e202211782.
- 87D. Zhu, T. Mu, Z.-L. Li, H.-Y. Luo, R.-F. Cao, X.-S. Xue, Z.-M. Chen, Angew. Chem. Int. Ed. 2024, 63, e202318625.
- 88X.-Y. Zhang, D. Zhu, R.-F. Cao, Y.-X. Huo, T.-M. Ding, Z.-M. Chen, Nat. Commun. 2024, 15, 9929.
- 89C. Xu, B. Ma, Q. Shen, Angew. Chem. Int. Ed. 2014, 53, 9316–9320.
- 90J. Mestre, M. Bernús, S. Castillón, O. Boutureira, J. Org. Chem. 2022, 87, 10791–10806.
- 91P. Casasús, J. Mestre, M. Bernús, S. Castillón, O. Boutureira, Adv. Synth. Catal. 2023, 365, 3438–3443.
- 92J. B. Ernst, A. Rühling, B. Wibbeling, F. Glorius, Chem. - Eur. J. 2016, 22, 4400–4404.
- 93D. Wu, J. Qiu, P. G. Karmaker, H. Yin, F.-X. Chen, J. Org. Chem. 2018, 83, 1576–1583.
- 94M. Tingoli, R. Diana, B. Panunzi, Tetrahedron Lett. 2006, 47, 7529–7531.
- 95G. Gao, K. Xie, M. Shi, T. Gao, Z. Wang, C. Zhang, Z. Wang, Org. Biomol. Chem. 2024, 22, 7707–7714.
- 96H. Yang, Y. Chen, X. Xu, Z. Li, Synlett 2022, 34, 176–182.
- 97 Deposition Numbers 2404782 (for 1n), 2404780 (for 2e), 2404779 (for 2j), 2404793 (for 5f), 2434264 (for 5ak), 2404783 (for s1a), 2404784 (for 1a), 2404787 (for 1d), 2404788 (for 1l), 2404792 (for 1q), 2434250 (for 1af), and 2434251 (for 1ag) contain the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service.
- 98S. E. Denmark, E. Hartmann, D. J. P. Kornfilt, H. Wang, Nat. Chem. 2014, 6, 1056–1064.
- 99A. Matviitsuk, J. L. Panger, S. E. Denmark, Angew. Chem. Int. Ed. 2020, 59, 19796–19819.
- 100L. Liao, X. Zhao, Acc. Chem. Res. 2022, 55, 2439–2453.