Hopping Diffusion in Wiggling Nanopore Architecture of MOF Enabling Synergistic Equilibrium-Kinetic Separation of Fluorinated Propylene and Propane
Wei Xia
Key Laboratory of Biomass Chemical Engineering of Ministry of Education, College of Chemical and Biological Engineering, Zhejiang University, Hangzhou, Zhejiang, 310058 P.R. China
Search for more papers by this authorZhijie Zhou
Key Laboratory of Biomass Chemical Engineering of Ministry of Education, College of Chemical and Biological Engineering, Zhejiang University, Hangzhou, Zhejiang, 310058 P.R. China
Search for more papers by this authorCan Xia
Key Laboratory of Biomass Chemical Engineering of Ministry of Education, College of Chemical and Biological Engineering, Zhejiang University, Hangzhou, Zhejiang, 310058 P.R. China
Search for more papers by this authorCorresponding Author
Lihang Chen
Institute of Zhejiang University-Quzhou, Quzhou, Zhejiang, 324000 P.R. China
E-mail: [email protected]; [email protected]; [email protected]
Search for more papers by this authorLiangzheng Sheng
Key Laboratory of Biomass Chemical Engineering of Ministry of Education, College of Chemical and Biological Engineering, Zhejiang University, Hangzhou, Zhejiang, 310058 P.R. China
Search for more papers by this authorCorresponding Author
Fang Zheng
Institute of Zhejiang University-Quzhou, Quzhou, Zhejiang, 324000 P.R. China
E-mail: [email protected]; [email protected]; [email protected]
Search for more papers by this authorZhiguo Zhang
Key Laboratory of Biomass Chemical Engineering of Ministry of Education, College of Chemical and Biological Engineering, Zhejiang University, Hangzhou, Zhejiang, 310058 P.R. China
Institute of Zhejiang University-Quzhou, Quzhou, Zhejiang, 324000 P.R. China
Search for more papers by this authorQiwei Yang
Key Laboratory of Biomass Chemical Engineering of Ministry of Education, College of Chemical and Biological Engineering, Zhejiang University, Hangzhou, Zhejiang, 310058 P.R. China
Institute of Zhejiang University-Quzhou, Quzhou, Zhejiang, 324000 P.R. China
Search for more papers by this authorQilong Ren
Key Laboratory of Biomass Chemical Engineering of Ministry of Education, College of Chemical and Biological Engineering, Zhejiang University, Hangzhou, Zhejiang, 310058 P.R. China
Institute of Zhejiang University-Quzhou, Quzhou, Zhejiang, 324000 P.R. China
Search for more papers by this authorCorresponding Author
Zongbi Bao
Key Laboratory of Biomass Chemical Engineering of Ministry of Education, College of Chemical and Biological Engineering, Zhejiang University, Hangzhou, Zhejiang, 310058 P.R. China
Institute of Zhejiang University-Quzhou, Quzhou, Zhejiang, 324000 P.R. China
E-mail: [email protected]; [email protected]; [email protected]
Search for more papers by this authorWei Xia
Key Laboratory of Biomass Chemical Engineering of Ministry of Education, College of Chemical and Biological Engineering, Zhejiang University, Hangzhou, Zhejiang, 310058 P.R. China
Search for more papers by this authorZhijie Zhou
Key Laboratory of Biomass Chemical Engineering of Ministry of Education, College of Chemical and Biological Engineering, Zhejiang University, Hangzhou, Zhejiang, 310058 P.R. China
Search for more papers by this authorCan Xia
Key Laboratory of Biomass Chemical Engineering of Ministry of Education, College of Chemical and Biological Engineering, Zhejiang University, Hangzhou, Zhejiang, 310058 P.R. China
Search for more papers by this authorCorresponding Author
Lihang Chen
Institute of Zhejiang University-Quzhou, Quzhou, Zhejiang, 324000 P.R. China
E-mail: [email protected]; [email protected]; [email protected]
Search for more papers by this authorLiangzheng Sheng
Key Laboratory of Biomass Chemical Engineering of Ministry of Education, College of Chemical and Biological Engineering, Zhejiang University, Hangzhou, Zhejiang, 310058 P.R. China
Search for more papers by this authorCorresponding Author
Fang Zheng
Institute of Zhejiang University-Quzhou, Quzhou, Zhejiang, 324000 P.R. China
E-mail: [email protected]; [email protected]; [email protected]
Search for more papers by this authorZhiguo Zhang
Key Laboratory of Biomass Chemical Engineering of Ministry of Education, College of Chemical and Biological Engineering, Zhejiang University, Hangzhou, Zhejiang, 310058 P.R. China
Institute of Zhejiang University-Quzhou, Quzhou, Zhejiang, 324000 P.R. China
Search for more papers by this authorQiwei Yang
Key Laboratory of Biomass Chemical Engineering of Ministry of Education, College of Chemical and Biological Engineering, Zhejiang University, Hangzhou, Zhejiang, 310058 P.R. China
Institute of Zhejiang University-Quzhou, Quzhou, Zhejiang, 324000 P.R. China
Search for more papers by this authorQilong Ren
Key Laboratory of Biomass Chemical Engineering of Ministry of Education, College of Chemical and Biological Engineering, Zhejiang University, Hangzhou, Zhejiang, 310058 P.R. China
Institute of Zhejiang University-Quzhou, Quzhou, Zhejiang, 324000 P.R. China
Search for more papers by this authorCorresponding Author
Zongbi Bao
Key Laboratory of Biomass Chemical Engineering of Ministry of Education, College of Chemical and Biological Engineering, Zhejiang University, Hangzhou, Zhejiang, 310058 P.R. China
Institute of Zhejiang University-Quzhou, Quzhou, Zhejiang, 324000 P.R. China
E-mail: [email protected]; [email protected]; [email protected]
Search for more papers by this authorGraphical Abstract
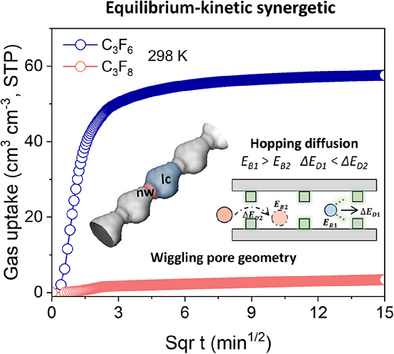
To address a critical challenge in electronic specialty gas (ESG) purification, we herein present a zirconium-based MOF with “wiggling pore geometry” featuring alternating narrow apertures and enlarged cavities. This architecture establishes an equilibrium-kinetic synergistic separation mechanism: narrow windows (nw) kinetically hinder C3F8 diffusion (DC3F6/DC3F8 ≈ 450), while F···H interactions in cavities thermodynamically favor C3F6 adsorption.
Abstract
The separation of octafluoropropane (C3F8) from hexafluoropropylene (C3F6) is an industrially important yet challenging process due to their similar physicochemical properties and stringent purity demands in industrial applications. Herein, we address this task through precise pore architecture in a zirconium-based metal-organic framework (Zr-PMA), which exhibits unique “wiggling nanopores” with narrow windows and large cavities. The narrow windows act as diffusion barriers, selectively restricting C3F8 transport, while the large cavities provide strong adsorption sites for C3F6, enabling an equilibrium-kinetic synergistic separation. This dual functionality results in a ∼450-fold difference in diffusion rates and exceptional kinetic selectivity for C3F6 over C3F8, as demonstrated by adsorption isotherms, time-resolved kinetics, and dynamic breakthrough experiments. Theoretical calculations coupled with in situ spectroscopy elucidate the pore geometry-dependent hopping diffusion mechanism responsible for the separation. This work establishes wiggling pore geometry as a versatile paradigm for advanced adsorbents targeting energy-efficient separations of structurally similar fluorocarbon mixtures.
Conflict of Interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Supporting Information
| Filename | Description |
|---|---|
| anie202503505-sup-0001-SupMat.pdf3.9 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1J. R. Clark, J. Chem. Educ. 2006, 83, 857.
- 2C. C. Allgood, J. Fluorine Chem. 2003, 122, 105–112.
- 3M. B. Chang, J.-S. Chang, Ind. Eng. Chem. Res. 2006, 45, 4101–4109.
- 4W. R. Dolbier Jr, J. Fluorine Chem. 2005, 126, 157–163.
- 5P. B. Leezenberg, T. C. Reiley, G. W. Tyndall, J. Vac. Sci. Technol., A 1999, 17, 275–281.
- 6J. Mühle, A. L. Ganesan, B. R. Miller, P. Salameh, C. Harth, B. Greally, M. Rigby, L. Porter, L. Steele, C. Trudinger, Atmos. Chem. Phys. 2010, 10, 5145–5164.
- 7G. Villalba, R. U. Ayres, H. Schroder, J. Ind. Ecol. 2007, 11, 85–101.
- 8X. Cao, R. Liu, Y. Lu, S. Jia, X. Yuan, Sep. Purif. Technol. 2021, 279, 119813.
- 9S. U. Rege, J. Padin, R. T. Yang, AIChE J. 1998, 44, 799–809.
- 10H. G. Karge, J. Weitkamp, Adsorption and Diffusion, Springer Science & Business Media, Berlin 2008.
- 11Y. Su, J.-J. Zheng, K. Otake, N. Hosono, S. Kitagawa, C. Gu, Acc. Chem. Res. 2024, 24, 3455–3464.
- 12J. Y. Lin, Science 2016, 353, 121–122.
- 13Q. M. Wang, D. Shen, M. Bülow, M. L. Lau, S. Deng, F. R. Fitch, N. O. Lemcoff, J. Semanscin, Microporous Mesoporous Mater. 2002, 55, 217–230.
- 14H. Zeng, M. Xie, T. Wang, R.-J. Wei, X.-J. Xie, Y. Zhao, W. Lu, D. Li, Nature 2021, 595, 542–548.
- 15L. Li, L. Guo, D. H. Olson, S. Xian, Z. Zhang, Q. Yang, K. Wu, Y. Yang, Z. Bao, Q. Ren, J. Li, Science 2022, 377, 335–339.
- 16S. Du, J. Huang, M. R. Ryder, L. L. Daemen, C. Yang, H. Zhang, P. Yin, Y. Lai, J. Xiao, S. Dai, B. Chen, Nat. Commun. 2023, 14, 1197.
- 17S. M. Kuznicki, V. A. Bell, S. Nair, H. W. Hillhouse, R. M. Jacubinas, C. M. Braunbarth, B. H. Toby, M. Tsapatsis, Nature 2001, 412, 720–724.
- 18J. Zhao, S. H. Mousavi, G. Xiao, A. H. Mokarizadeh, T. Moore, K. Chen, Q. Gu, R. Singh, A. Zavabeti, J. Z. Liu, P. A. Webley, G. K. Li, J. Am. Chem. Soc. 2021, 143, 15195–15204.
- 19Y. Yuan, Y. Wang, X. Zhang, W. Li, G. Hao, L. Han, A. Lu, Angew. Chem. Int. Ed. 2021, 60, 19063–19067.
- 20L.-P. Guo, R.-S. Liu, J. Qian, G.-P. Hao, J. Guo, H. Wu, F. Wang, A.-H. Lu, Nat. Chem. Eng. 2024, 1, 411–420.
10.1038/s44286-024-00075-9 Google Scholar
- 21A. W. Anjum, L. Zhu, J. Huang, N. Liao, S. Du, Z. Li, C. Yang, J. Xiao, Chem. Bio. Eng. 2024, 1, 960–969.
- 22R. C. Bansal, M. Goyal, in Activated Carbon Adsorption, Taylor & Franics Group CRC Press, Boca Ration 2005.
10.1201/9781420028812 Google Scholar
- 23J. Shang, G. Li, R. Singh, Q. Gu, K. M. Nairn, T. J. Bastow, N. Medhekar, C. M. Doherty, A. J. Hill, J. Z. Liu, P. A. Webley, J. Am. Chem. Soc. 2012, 134, 19246–19253.
- 24S. Kitagawa, R. Kitaura, S. Noro, Angew. Chem. Int. Ed. 2004, 43, 2334–2375.
- 25J.-R. Li, R. J. Kuppler, H.-C. Zhou, Chem. Soc. Rev. 2009, 38, 1477–1504.
- 26S. Wuttke, Angew. Chem. Int. Ed. 2019, 58, 14024–14024.
- 27E. D. Bloch, W. L. Queen, R. Krishna, J. M. Zadrozny, C. M. Brown, J. R. Long, Science 2012, 335, 1606–1610.
- 28J. Zheng, R. S. Vemuri, L. Estevez, P. K. Koech, T. Varga, D. M. Camaioni, T. A. Blake, B. P. McGrail, R. K. Motkuri, J. Am. Chem. Soc. 2017, 139, 10601–10604.
- 29A. Cadiau, K. Adil, P. Bhatt, Y. Belmabkhout, M. Eddaoudi, Science 2016, 353, 137–140.
- 30Z. Bao, J. Wang, Z. Zhang, H. Xing, Q. Yang, Y. Yang, H. Wu, R. Krishna, W. Zhou, B. Chen, Q. Ren, Angew. Chem. Int. Ed. 2018, 130, 16252–16257.
- 31D.-D. Zhou, P. Chen, C. Wang, S.-S. Wang, Y. Du, H. Yan, Z.-M. Ye, C.-T. He, R.-K. Huang, Z.-W. Mo, Nat. Mater. 2019, 18, 994–998.
- 32Q. Dong, Y. Huang, J. Wan, Z. Lu, Z. Wang, C. Gu, J. Duan, J. Bai, J. Am. Chem. Soc. 2023, 145, 8043–8051.
- 33X.-J. Xie, Q.-Y. Cao, Z.-H. Zhang, M.-Y. Zhou, H. Zeng, W. Lu, D. Li, Chem. Bio. Eng. 2024, 1, 150–156.
- 34W. Xia, Z. Zhou, L. Sheng, L. Chen, F. Shen, F. Zheng, Z. Zhang, Q. Yang, Q. Ren, Z. Bao, Nat. Commun. 2024, 15, 8716.
- 35W. Xia, Y. Yang, L. Sheng, Z. Zhou, L. Chen, Z. Zhang, Z. Zhang, Q. Yang, Q. Ren, Z. Bao, Sci. Adv. 2024, 10, eadj6473.
- 36M. Zheng, W. Xue, T. Yan, Z. Jiang, Z. Fang, H. Huang, C. Zhong, Angew. Chem. Int. Ed. 2024, 63, e202401770.
- 37W. Xia, Z. Zhou, L. Sheng, L. Chen, F. Zheng, Z. Zhang, Q. Yang, Q. Ren, Z. Bao, Sci. Bull. 2025, 70, 232–240.
- 38X. Huang, F. Chen, H. Sun, L. Yang, Q. Yang, Z. Zhang, Y. Yang, Q. Ren, Z. Bao, J. Am. Chem. Soc. 2024, 146, 617–626.
- 39Y. Wang, N. Huang, X. Zhang, H. He, R. Huang, Z. Ye, Y. Li, D. Zhou, P. Liao, X. Chen, J. Zhang, Angew. Chem. Int. Ed. 2019, 58, 7692–7696.
- 40Q. Ding, Z. Zhang, C. Yu, P. Zhang, J. Wang, X. Cui, C.-H. He, S. Deng, H. Xing, Sci. Adv. 2020, 6, eaaz4322.
- 41Q. Liu, S. G. Cho, J. Hilliard, T. Wang, S. Chien, L. Lin, A. C. Co, C. R. Wade, Angew. Chem. Int. Ed. 2023, 62, e202218854.
- 42J. Peng, H. Wang, D. H. Olson, Z. Li, J. Li, Chem. Commun. 2017, 53, 9332–9335.
- 43Y. Xiao, Y. Chen, W. Wang, H. Yang, A. N. Hong, X. Bu, P. Feng, J. Am. Chem. Soc. 2023, 145, 10980–10986.
- 44J. Li, X. Han, X. Kang, Y. Chen, S. Xu, G. L. Smith, E. Tillotson, Y. Cheng, L. J. McCormick McPherson, S. J. Teat, S. Rudić, A. J. Ramirez-Cuesta, S. J. Haigh, M. Schröder, S. Yang, Angew. Chem. Int. Ed. 2021, 60, 15541–15547.
- 45A. E. Khudozhitkov, S. S. Arzumanov, D. I. Kolokolov, D. Freude, A. G. Stepanov, Phys. Chem. Chem. Phys. 2020, 22, 5976–5984.
- 46M. P. M. Poschmann, K. P. Lillerud, N. Stock, Chem. Eur. J. 2023, 29, e202301760.
- 47J. Xiao, J. Wei, Chem. Eng. Sci. 1992, 47, 1123–1141.
- 48L. Dewis, R. Crouch, D. Russell, C. Butts, Magn. Reson. Chem. 2019, 57, 1143–1149.
- 49S. Marbach, D. S. Dean, L. Bocquet, Nat. Phys. 2018, 14, 1108–1113.
- 50J. Cui, Z. Zhang, L. Yang, J. Hu, A. Jin, Z. Yang, Y. Zhao, B. Meng, Y. Zhou, J. Wang, Y. Su, J. Wang, X. Cui, H. Xing, Science 2024, 383, 179–183.