Highly Selective Oxygen Electroreduction to Hydrogen Peroxide on Sulfur-Doped Mesoporous Carbon
Graphical Abstract
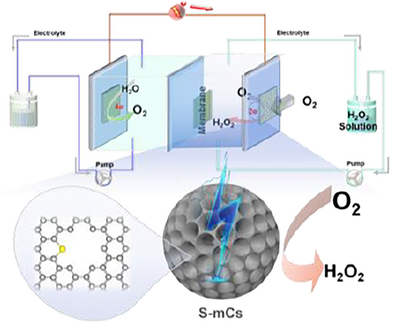
Vertically aligned sulfur-doped mesoporous carbon spheres achieve outstanding 2e⁻-ORR performance with >99% H₂O₂ selectivity, −3.5 mA cm⁻2 current density, and 25 mol g⁻¹ h⁻¹ yield. The sp2/sp3 hybrid network synergized with S-induced charge redistribution creates electron-deficient hotspots for *OOH stabilization, combining metal-like efficiency, chemical robustness, and cost-effectiveness. This structural–electronic paradigm opens a new way for sustainable H₂O₂ electrosynthesis.
Abstract
As a paradigm-shifting material platform in energy catalysis, precisely engineered ordered mesoporous carbon spheres emerge as supreme metal-free electrocatalysts, outperforming conventional carbon-based counterparts through synergistic structural and electronic innovations. Herein, we architecturally design vertically aligned cylindrical mesoporous carbon spheres with atomic-level sulfur doping (S-mC) that establish unprecedented performance benchmarks in the two-electron oxygen reduction reaction (2e−-ORR) to hydrogen peroxide. Systematic comparative studies reveal that the S-mC catalysts achieve exceptional H2O2 selectivity (>99%) and activity at current density of −3.5 mA cm−2, surpassing state-of-the-art metal-free catalysts in current density. Impressively, the optimized S-mC electrocatalyst in a flow cell device achieves an exceptional H2O2 yield of 25 mol gcatalyst−1 h−1. The carbon matrix's unique sp2/sp3 hybrid network coupled with S-induced charge redistribution generates electron-deficient hotspots that selectively stabilize *OOH intermediates, as evidenced by in situ spectroscopic characterization and DFT calculations. This structural–electronic synergy endows the carbon framework with metal-like catalytic efficiency while maintaining inherent advantages of chemical robustness and cost-effectiveness. The marriage of S-doping engineering with mesoscopic pore architecture control opens a new way for developing efficient carbon-based electrocatalysts for oxygen selective reduction to H2O2.
Conflict of Interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
Research data are not shared.