Modular Access to Arylethylamines Enabled by Ni-Catalyzed Markovnikov-Selective Hydroarylation of Allylic Amines
Hai-Yu Wu
State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, 345 Lingling Road, Shanghai, 200032 P.R. China
Search for more papers by this authorCorresponding Author
Ming Joo Koh
Department of Chemistry, National University of Singapore, Singapore, 117544 Republic of Singapore
E-mail: [email protected]; [email protected]; [email protected]
Search for more papers by this authorCorresponding Author
Zi-Chao Wang
State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, 345 Lingling Road, Shanghai, 200032 P.R. China
E-mail: [email protected]; [email protected]; [email protected]
Search for more papers by this authorCorresponding Author
Shi-Liang Shi
State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, 345 Lingling Road, Shanghai, 200032 P.R. China
E-mail: [email protected]; [email protected]; [email protected]
Search for more papers by this authorHai-Yu Wu
State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, 345 Lingling Road, Shanghai, 200032 P.R. China
Search for more papers by this authorCorresponding Author
Ming Joo Koh
Department of Chemistry, National University of Singapore, Singapore, 117544 Republic of Singapore
E-mail: [email protected]; [email protected]; [email protected]
Search for more papers by this authorCorresponding Author
Zi-Chao Wang
State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, 345 Lingling Road, Shanghai, 200032 P.R. China
E-mail: [email protected]; [email protected]; [email protected]
Search for more papers by this authorCorresponding Author
Shi-Liang Shi
State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, 345 Lingling Road, Shanghai, 200032 P.R. China
E-mail: [email protected]; [email protected]; [email protected]
Search for more papers by this authorGraphical Abstract
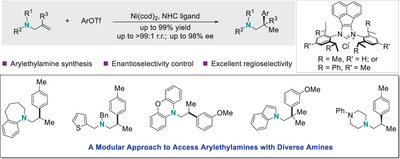
We describe a modular and efficient nickel-catalyzed Markovnikov-selective hydroarylation of readily available allylic amines, affording various valuable arylethylamines with exclusive regiocontrol. The use of NHC─Ni catalyst was crucial to achieve high efficiency and selectivity. In particular, with bulky chiral NHC ligands, enantioenriched arylethylamines were prepared in high efficiency with excellent regio- and enantioselectivities.
Abstract
Arylethylamines are prevalent structural skeletons in bioactive molecules and have significant interest within the organic chemistry community. We report here a modular and efficient nickel-catalyzed Markovnikov-selective hydroarylation of readily available allylic amines, delivering a wide variety of valuable arylethylamines with complete regiocontrol under mild conditions. Key to the success of this protocol is the employment of bulky N-heterocyclic carbenes (NHCs) as ligands. Furthermore, the use of chiral NHC ligands enables straightforward access to enantioenriched arylethylamines with excellent regio- and enantioselectivities.
Conflict of Interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the supporting information of this article.
Supporting Information
| Filename | Description |
|---|---|
| anie202503126-sup-0001-SuppMat.pdf16.8 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1J. W. Dalley, B. J. Everitt, Semin. Cell. Dev. Biol. 2009, 64, 403–410.
- 2S. Obeng, T. Hiranita, F. León, L. R. McMahon, C. R. McCurdy, J. Med. Chem. 2021, 64, 6523–6548.
- 3R. R. Gainetdinov, M. C. Hoener, M. D. Berry, Pharmacol. Rev. 2018, 70, 549–620.
- 4E. A. Ilardi, E. Vitaku, J. T. Njardarson, J. Med. Chem. 2014, 57, 2832–2842.
- 5P. Jones, K. Wilcoxen, M. Rowley, C. Toniatti, J. Med. Chem. 2015, 58, 3302–3314.
- 6J. Wang, T. Hou, J. Chem. Inf. Model. 2010, 50, 55–67.
- 7J. Zhang, B. Xiong, X. Zhen, A. Zhang, Med. Res. Rev. 2009, 29, 272–294.
- 8A. Zhang, J. L. Neumeyer, R. J. Baldessarini, Chem. Rev. 2007, 107, 274–302.
- 9https://bpb-us-e2.wpmucdn.com/sites.arizona.edu/dist/9/130/files/2024/07/2023Top200SmallMoleculePosterV6.pdf.
- 10V. Pozhydaiev, C. Muller, J. Moran, D. Lebœuf, Angew. Chem. Int. Ed. 2023, 135, e202309289.
10.1002/ange.202309289 Google Scholar
- 11A. Cabré, X. Verdaguer, A. Riera, Chem. Rev. 2022, 122, 269–339.
- 12C.-Y. Huang, A. G. Doyle, J. Am. Chem. Soc. 2012, 134, 9541–9544.
- 13X. Hu, I. Cheng-Sánchez, S. Cuesta-Galisteo, C. Nevado, J. Am. Chem. Soc. 2023, 145, 6270–6279.
- 14W. L. Williams, N. E. Gutiérrez-Valencia, A. G. Doyle, J. Am. Chem. Soc. 2023, 145, 24175–24183.
- 15Y.-Z. Wang, Z.-H. Wang, I.-L. Eshel, B. Sun, D. Liu, Y.-C. Gu, A. Milo, T.-S. Mei, Nat. Commun. 2023, 14, 2322.
- 16G. S. Kumar, C. Zhu, R. Kancherla, P. S. Shinde, M. Rueping, ACS Catal. 2023, 13, 8813–8820.
- 17S. Samanta, P. Biswas, B. C. O'Bannon, D. C. Powers, Angew. Chem. Int. Ed. 2024, 136, e202406335.
- 18A. Millet, D. Dailler, P. Larini, O. Baudoin, Angew. Chem. Int. Ed. 2014, 53, 2678–2682.
- 19E. Brunard, V. Boquet, E. Van Elslande, T. Saget, P. Dauban, J. Am. Chem. Soc. 2021, 143, 6407–6412.
- 20E. Brunard, V. Boquet, T. Saget, E. D. Sosa Carrizo, M. Sircoglou, P. Dauban, J. Am. Chem. Soc. 2024, 146, 5843–5854.
- 21M. Utsunomiya, R. Kuwano, M. Kawatsura, J. F. Hartwig, J. Am. Chem. Soc. 2003, 125, 5608–5609.
- 22M. Utsunomiya, J. F. Hartwig, J. Am. Chem. Soc. 2004, 126, 2702–2703.
- 23T. M. Nguyen, N. Manohar, D. A. Nicewicz, Angew. Chem. Int. Ed. 2014, 53, 6198–6201.
- 24S. M. Bronner, R. H. Grubbs, Chem. Sci. 2014, 5, 101–106.
- 25A. J. Musacchio, B. C. Lainhart, X. Zhang, S. G. Naguib, T. C. Sherwood, R. R. Knowles, Science 2017, 355, 727–730.
- 26N. Wagner Carlberg, T. Rovis, J. Am. Chem. Soc. 2022, 144, 22426–22432.
- 27C. Xu, C. W. Muir, A. G. Leach, A. R. Kennedy, A. J. Watson, Angew. Chem. Int. Ed. 2018, 130, 11544–11547.
- 28S. Zhu, S. L. Buchwald, J. Am. Chem. Soc. 2014, 136, 15913–15916.
- 29H. Jiang, A. Studer, Chem. Soc. Rev. 2020, 49, 1790–1811.
- 30Y. Kwon, Q. Wang, Chem.-Asian. J. 2022, 17, e202200215.
- 31A. Lerchen, T. Knecht, C. G. Daniliuc, F. Glorius, Angew. Chem. Int. Ed. 2016, 55, 15166–15170.
- 32D. Wang, L. Wu, F. Wang, X. Wan, P. Chen, Z. Lin, G. Liu, J. Am. Chem. Soc. 2017, 139, 6811–6814.
- 33T. Kang, N. Kim, P. T. Cheng, H. Zhang, K. Foo, K. M. Engle, J. Am. Chem. Soc. 2021, 143, 13962–13970.
- 34L. Xie, S. Wang, L. Zhang, L. Zhao, C. Luo, L. Mu, X. Wang, C. Wang, Nat. Commun. 2021, 12, 6280–6289.
- 35H. Jiang, X. Yu, C. G. Daniliuc, A. Studer, Angew. Chem. Int. Ed. 2021, 60, 14399–14404.
- 36X.-K. Qi, M.-J. Zheng, C. Yang, Y. Zhao, L. Guo, W. Xia, J. Am. Chem. Soc. 2023, 145, 16630–16641.
- 37A. Bunescu, Y. Abdelhamid, M. J. Gaunt, Nature 2021, 598, 597–603.
- 38Y. Cai, S. Chatterjee, T. Ritter, J. Am. Chem. Soc. 2023, 145, 13542–13548.
- 39T. M. Monos, R. C. McAtee, C. R. Stephenson, Science 2018, 361, 1369–1373.
- 40E. A. Noten, C. H. Ng, R. M. Wolesensky, C. R. Stephenson, Nat. Chem. 2024, 16, 599–606.
- 41C. Hervieu, M. S. Kirillova, Y. Hu, S. Cuesta-Galisteo, E. Merino, C. Nevado, Nat. Chem. 2024, 16, 607–614.
- 42R. Kubiak, I. Prochnow, S. Doye, Angew. Chem. Int. Ed. 2010, 49, 2626–2629.
- 43R. C. DiPucchio, K. E. Lenzen, P. Daneshmand, M. B. Ezhova, L. L. Schafer, J. Am. Chem. Soc. 2021, 143, 11243–11250.
- 44R. C. DiPucchio, S. C. Rosca, L. L. Schafer, J. Am. Chem. Soc. 2022, 144, 11459–11481.
- 45Y. Wang, Y. He, S. Zhu, Acc. Chem. Res. 2022, 55, 3519–3536.
- 46M. Xu, W. Xu, M. Ye, Eur. J. Org. Chem. 2024, e202401000.
- 47S. Teng, J. S. Zhou, Chem. Soc. Rev. 2022, 51, 1592–1607.
- 48Y. Nakao, Synthesis 2011, 2011, 3209–3219.
- 49Z. Dong, Z. Ren, S. J. Thompson, Y. Xu, G. Dong, Chem. Rev. 2017, 117, 9333–9403.
- 50A. J. Boyington, C. P. Seath, A. M. Zearfoss, Z. Xu, N. T. Jui, J. Am. Chem. Soc. 2019, 141, 4147–4153.
- 51R. A. Aycock, D. B. Vogt, N. T. Jui, Chem. Sci. 2017, 8, 7998–8003.
- 52Y. He, C. Du, J. Han, J. Han, C. Zhu, J. Xie, Chin. J. Chem. 2022, 40, 1546–1552.
- 53W. Zhao, B. J. Li, J. Am. Chem. Soc. 2023, 145, 6861–6870.
- 54S. Raje, T. Sheikh Mohammad, G. de Ruiter, J. Org. Chem. 2024, 89, 4319–4325.
- 55S. Krompiec, M. Pigulla, M. Krompiec, B. Marciniec, D. Chadyniak, J. Mol. Catal. A: Chem. 2005, 237, 17–25.
- 56P. Bujak, S. Krompiec, J. Malarz, M. Krompiec, M. Filapek, W. Danikiewicz, K. Magdalena, G. Katarzyna, I. Grudzka, Tetrahedron 2010, 66, 5972–5981.
- 57B. M. Trost, D. R. Fandrick, T. Brodmann, D. T. Stiles, Angew. Chem. Int. Ed. 2007, 46, 6123–6125.
- 58X.-H. Zhao, D.-L. Liu, H. Guo, Y.-G. Liu, W.-B. Zhang, J. Am. Chem. Soc. 2011, 133, 19354–19357.
- 59X.-S. Wu, Y. Chen, M.-B. Li, M.-G. Zhou, S.-K. Tian, J. Am. Chem. Soc. 2012, 134, 14694–14697.
- 60Y. Cai, X.-T. Yang, S.-Q. Zhang, F. Li, Y.-Q. Li, L.-X. Ruan, X. Hong, S.-L. Shi, Angew. Chem. Int. Ed. 2018, 57, 1376–1380.
- 61Y. Cai, J.-W. Zhang, F. Li, J.-M. Liu, S.-L. Shi, ACS Catal. 2019, 9, 1–6.
- 62D. Shen, Y. Xu, S.-L. Shi, J. Am. Chem. Soc. 2019, 141, 14938–14945.
- 63J.-M. Liu, X. Ma, G. Chen, W. Wan, Z. Li, Y. Xu, D. Zhang, S.-L. Shi, Sci. Bull. 2025, 70, 674–682.
- 64M. N. Hopkinson, C. Richter, M. Schedler, F. Glorius, Nature 2014, 510, 485–496.
- 65S. Diez-Gonzalez, N. Marion, S. P. Nolan, Chem. Rev. 2009, 109, 3612–3676.
- 66B. C. Lee, C.-F. Liu, L. Q. H. Lin, K. Z. Yap, N. Song, C. H. M. Ko, P. H. Chan, M. J. Koh, Chem. Soc. Rev. 2023, 52, 2946–2991.
- 67D. Foster, S. M. Borhanuddin, R. Dorta, Dalton Trans. 2021, 50, 17467–17477.
- 68Z.-C. Wang, X. Luo, J.-W. Zhang, C.-F. Liu, M. J. Koh, S.-L. Shi, Nat. Catal. 2023, 6, 1087–1097.
- 69C.-F. Liu, Z.-C. Wang, X. Luo, J. Lu, C. H. M. Ko, S.-L. Shi, M. J. Koh, Nat. Catal. 2022, 5, 934–942.
- 70Z.-C. Wang, J.-W. Zhang, M. J. Koh, S.-L. Shi, Angew. Chem. Int. Ed. 2023, 135, e202310203.
- 71B. Jiang, J.-M. Liu, S.-L. Shi, ACS Catal. 2023, 13, 6068–6075.
- 72J.-B. Ma, X. Zhao, D. Zhang, S.-L. Shi, J. Am. Chem. Soc. 2022, 144, 13643–13651.
- 73Y. Cai, X. Ye, S. Liu, S.-L. Shi, Angew. Chem. Int. Ed. 2019, 131, 13567–13571.
- 74W.-B. Zhang, X.-T. Yang, J.-B. Ma, Z.-M. Su, S.-L. Shi, J. Am. Chem. Soc. 2019, 141, 5628–5634.
- 75C.-F. Liu, X. Luo, H. Wang, M. J. Koh, J. Am. Chem. Soc. 2021, 143, 9498–9506.
- 76Y. Wang, Y. He, S. Zhu, Acc. Chem. Res. 2023, 56, 3475–3491.
- 77Y. Li, G. Yin, Acc. Chem. Res. 2023, 56, 3246–3259.
- 78C. Romano, R. Martin, Nat. Rev. Chem. 2024, 1–18.
- 79C. Alamillo-Ferrer, G. Hutchinson, J. Burés, Nat. Rev. Chem. 2023, 7, 26–34.