Tandem Biocatalysis to Generate Hydrogen Sulfide and Promote Endogenous Antioxidant Response
Graphical Abstract
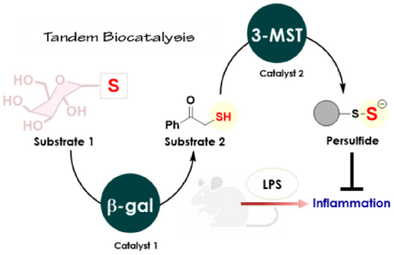
A strategy where we integrated spatially and temporally controlled catalysis in one system to promote antioxidant response is reported. We developed a glycoconjugate that is cleaved sequentially by β-galactosidase (β-gal) and 3-mercaptopyruvate sulfurtransferase (3-MST) to produce potent antioxidants persulfide and hydrogen sulfide, and this compound was found to mitigate inflammation in an animal model.
Abstract
Promoting cellular protective responses during oxidative stress conditions through the generation of antioxidant persulfide (RS-SH) and hydrogen sulfide (H2S) has tremendous therapeutic potential. Here, we report a bioinspired glycoconjugate, a candidate for tandem biocatalysis and generates persulfide/ H2S in response to oxidative stress. The glycoconjugate is cleaved by β-galactosidase, an enzyme that is expressed during oxidative stress; the product of this reaction is a substrate for 3-mercaptopyruvate sulfurtransferase (3-MST), an enzyme that is involved in persulfide/ H2S biosynthesis. The catalytic systems are orthogonal to one another, and the glycoconjugate is efficiently cleaved by these enzymes to generate the potent antioxidant glutathione persulfide as well as H2S. We demonstrate the efficacy of this conjugate in mitigating inflammation in the brain in an animal model. Together, using rationally designed substrates and fully catalytic steps, we leverage tandem biocatalysis to direct the generation of persulfide/ H2S, and promote cells’ own antioxidant response.
Conflict of Interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.