Reductive Catalytic Fractionation of Lignocellulose Toward Propyl- or Propenyl-Substituted Monomers and Mechanistic Understanding
Graphical Abstract
Abstract
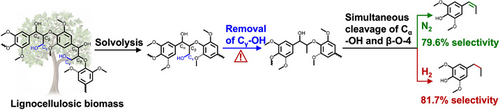
Reductive catalytic fractionation (RCF) is a promising technology that can selectively extract lignin in biomass and depolymerize it. Here, we prepared one low Ru loading catalyst (Ru0.8/C) for RCF of biomass to selectively produce different lignin oils (both monomers and oligomers) under different reaction atmospheres. The yield of phenolic monomers reached 46.0wt.% (rich in 4-propylguaiacol and 4-propylsyringol) in the RCF of birch wood under high H2 pressure and using methanol as the solvent. But, under N2 atmosphere the dominant monomers shifted to 4-(prop-1-enyl)guaiacol and 4-(prop-1-enyl)syringol (79.6% selectivity) with 35.8wt.% yield of total monomers using the same catalyst. The developed catalyst can also transform native lignin in other biomasses such as pine and corn stover. Mechanistic investigation using different model compounds and deuteration indicates that removal of the Cγ─OH in methanol-extracted lignin fragments occurred before obtaining the monomeric lignin fragment, and the Cα─OH and β─O─4 bonds were then cleaved simultaneously to form 4-(prop-1-enyl) substituted monomers. The results are distinct from the reported mechanism that the precursors of the propyl and prop-1-enyl substituted monomers are monolignols with removal of the Cγ─OH at the monomeric level. Thus, this work provided novel insights into the reaction pathway of (native) lignin depolymerization.
Conflict of Interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the Supporting Information of this article.