Unexpected Redox Role of WO3 in V2O5-WO3/TiO2 Catalysts for Selective Reduction of NO by Forming V–W Dinuclear Sites
Zhuocan Li
State Key Joint Laboratory of Environment Simulation and Pollution Control, Research Center for Eco-Environmental Sciences, Chinese Academy of Sciences, Beijing, 100085 China
College of Resources and Environment, University of Chinese Academy of Sciences, Beijing, 100049 China
Search for more papers by this authorRucheng Duan
State Key Joint Laboratory of Environment Simulation and Pollution Control, Research Center for Eco-Environmental Sciences, Chinese Academy of Sciences, Beijing, 100085 China
College of Resources and Environment, University of Chinese Academy of Sciences, Beijing, 100049 China
Search for more papers by this authorXin Wang
State Key Joint Laboratory of Environment Simulation and Pollution Control, Research Center for Eco-Environmental Sciences, Chinese Academy of Sciences, Beijing, 100085 China
College of Resources and Environment, University of Chinese Academy of Sciences, Beijing, 100049 China
Search for more papers by this authorYu Fu
State Key Joint Laboratory of Environment Simulation and Pollution Control, Research Center for Eco-Environmental Sciences, Chinese Academy of Sciences, Beijing, 100085 China
Search for more papers by this authorMeng Gao
State Key Joint Laboratory of Environment Simulation and Pollution Control, Research Center for Eco-Environmental Sciences, Chinese Academy of Sciences, Beijing, 100085 China
Search for more papers by this authorHongwei Li
Beijing Nuclear Magnetic Resonance Center, College of Chemistry and Molecular Engineering, Peking University, Beijing, 100871 China
Search for more papers by this authorCorresponding Author
Guangzhi He
State Key Joint Laboratory of Environment Simulation and Pollution Control, Research Center for Eco-Environmental Sciences, Chinese Academy of Sciences, Beijing, 100085 China
College of Resources and Environment, University of Chinese Academy of Sciences, Beijing, 100049 China
E-mail: [email protected]; [email protected]
Search for more papers by this authorCorresponding Author
Hong He
State Key Joint Laboratory of Environment Simulation and Pollution Control, Research Center for Eco-Environmental Sciences, Chinese Academy of Sciences, Beijing, 100085 China
College of Resources and Environment, University of Chinese Academy of Sciences, Beijing, 100049 China
Center for Excellence in Regional Atmospheric Environment, Institute of Urban Environment, Chinese Academy of Sciences, Xiamen, 361021 China
E-mail: [email protected]; [email protected]
Search for more papers by this authorZhuocan Li
State Key Joint Laboratory of Environment Simulation and Pollution Control, Research Center for Eco-Environmental Sciences, Chinese Academy of Sciences, Beijing, 100085 China
College of Resources and Environment, University of Chinese Academy of Sciences, Beijing, 100049 China
Search for more papers by this authorRucheng Duan
State Key Joint Laboratory of Environment Simulation and Pollution Control, Research Center for Eco-Environmental Sciences, Chinese Academy of Sciences, Beijing, 100085 China
College of Resources and Environment, University of Chinese Academy of Sciences, Beijing, 100049 China
Search for more papers by this authorXin Wang
State Key Joint Laboratory of Environment Simulation and Pollution Control, Research Center for Eco-Environmental Sciences, Chinese Academy of Sciences, Beijing, 100085 China
College of Resources and Environment, University of Chinese Academy of Sciences, Beijing, 100049 China
Search for more papers by this authorYu Fu
State Key Joint Laboratory of Environment Simulation and Pollution Control, Research Center for Eco-Environmental Sciences, Chinese Academy of Sciences, Beijing, 100085 China
Search for more papers by this authorMeng Gao
State Key Joint Laboratory of Environment Simulation and Pollution Control, Research Center for Eco-Environmental Sciences, Chinese Academy of Sciences, Beijing, 100085 China
Search for more papers by this authorHongwei Li
Beijing Nuclear Magnetic Resonance Center, College of Chemistry and Molecular Engineering, Peking University, Beijing, 100871 China
Search for more papers by this authorCorresponding Author
Guangzhi He
State Key Joint Laboratory of Environment Simulation and Pollution Control, Research Center for Eco-Environmental Sciences, Chinese Academy of Sciences, Beijing, 100085 China
College of Resources and Environment, University of Chinese Academy of Sciences, Beijing, 100049 China
E-mail: [email protected]; [email protected]
Search for more papers by this authorCorresponding Author
Hong He
State Key Joint Laboratory of Environment Simulation and Pollution Control, Research Center for Eco-Environmental Sciences, Chinese Academy of Sciences, Beijing, 100085 China
College of Resources and Environment, University of Chinese Academy of Sciences, Beijing, 100049 China
Center for Excellence in Regional Atmospheric Environment, Institute of Urban Environment, Chinese Academy of Sciences, Xiamen, 361021 China
E-mail: [email protected]; [email protected]
Search for more papers by this authorGraphical Abstract
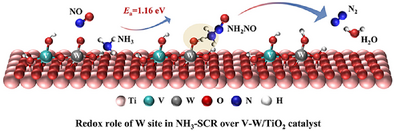
In the NH3-SCR reaction over V2O5-WO3/TiO2 catalyst, WO3 not only serves as an acidic promoter and structural regulator, but also plays a redox role. The WO3 directly participates in the oxidative activation of NH3, and hence explicitly exerts a redox effect by forming V–W dinuclear sites with V2O5.
Abstract
Promoters can greatly improve the activity of catalysts; however, the mechanism by which they interact with the primary catalyst to enhance catalytic activity is not always clear. It has been widely demonstrated that WO3 in V2O5-WO3/TiO2 catalysts acts as a promoter to enhance the surface acidity and regulate the dispersion of active V2O5 species, while it is believed that the redox process takes place entirely at the V sites in the selective catalytic reduction of NOx with NH3 (NH3-SCR). Here, by combining in situ spectroscopic measurements and density functional theory (DFT) calculations, we validate that the WO3 directly participates in the oxidative activation of NH3, and hence explicitly exerts a redox effect in NH3-SCR by forming V–W dinuclear sites with V2O5. This study sheds new light on a long-standing puzzle regarding the role of WO3 as a promoter and hence advances the understanding of the working principle of V2O5-WO3/TiO2 catalysts.
Conflict of Interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
Data available in article Supporting Information.
Supporting Information
| Filename | Description |
|---|---|
| anie202501957-supp-0001-SuppMat.docx29.4 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1W. Shan, Y. Yu, Y. Zhang, G. He, Y. Peng, J. Li, H. He, Catal. Today 2021, 376, 292–301.
- 2I. Hussain, A. A. Jalil, N. S. Hassan, M. Y. S. Hamid, J. Energy Chem. 2021, 62, 377–407.
- 3A. Kaszonyi, M. Hronec, G. Delahay, D. Ballivet-Tkatchenko, Appl. Catal., A 1999, 184, 103–113.
- 4C. Paun, S. Boghosian, V. Pârvulescu, P. Massiot, M. A. Centeno, P. Grange, V. I. Pârvulescu, Catal. Today 2004, 91-92, 33–37.
- 5W. G. Davenport, M. J. King, in Sulfuric Acid Manufacture. Elsevier, Oxford, 2006, p. 8.
- 6X. Wang, Y. Kang, J. Li, D. Li, Korean J. Chem. Eng. 2019, 36, 650–659.
- 7J. Cao, J. Xia, Y. Zhang, X. Liu, L. Bai, J. Xu, C.-A. Yang, S. Zheng, T. Yang, K. Tang, C. Zhang, C. Zhou, Fuel. 2021, 289, 119843.
- 8F. Rashidi, T. Sasaki, A. M. Rashidi, A. N. Kharat, K. J. Jozani, J. Catal. 2013, 299, 321–335.
- 9S. Eijsbouts, L. C. A. van den Oetelaar, J. N. Louwen, R. R. van Puijenbroek, K. G. C. van Leerdam, Ind. Eng. Chem. Res. 2007, 46, 3945–3954.
- 10Y. Okamoto, K. Ochiai, M. Kawano, T. Kubota, J. Catal. 2004, 222, 143–151.
- 11Y. Okamoto, S. Y. Ishihara, M. Kawano, M. Satoh, T. Kubota, J. Catal. 2003, 217, 12–22.
- 12P. Ratnasamy, H. Knozinger, J. Catal. 1978, 54, 155–165.
- 13Z. Li, M. Gao, Z. Lv, R. Duan, Y. Shan, H. Li, G. He, H. He, Environ. Sci. Technol. 2023, 57, 17577–17587.
- 14W. Y. Qu, H. Y. Yuan, Z. H. Ren, J. Z. Qi, D. R. Xu, J. X. Chen, L. W. Chen, H. G. Yang, Z. Ma, X. Liu, H. F. Wang, X. F. Tang, Angew. Chem., Int. Ed. 2022, 61, e202212703.
- 15Y. Inomata, H. Kubota, S. Hata, E. Kiyonaga, K. Morita, K. Yoshida, N. Sakaguchi, T. Toyao, K. I. Shimizu, S. Ishikawa, W. Ueda, M. Haruta, T. Murayama, Nat. Commun. 2021, 12, 11.
- 16J.-K. Lai, N. R. Jaegers, B. M. Lis, M. Guo, M. E. Ford, E. Walter, Y. Wang, J. Z. Hu, I. E. Wachs, ACS Catal. 2021, 11, 12096–12111.
- 17S. W. Jeon, I. Song, H. Lee, D. H. Kim, Chemosphere 2021, 275, 130105.
- 18W. Y. Qu, X. Fang, Z. H. Ren, J. X. Chen, X. Liu, Z. Ma, X. F. Tang, Environ. Sci. Technol. 2023, 57, 7858–7866.
- 19N. R. Jaegers, J.-K. Lai, Y. He, E. Walter, D. A. Dixon, M. Vasiliu, Y. Chen, C. Wang, M. Y. Hu, K. T. Mueller, I. E. Wachs, Y. Wang, J. Z. Hu, Angew. Chem., Int. Ed. 2019, 58, 12609–12616.
- 20J.-K. Lai, I. E. Wachs, ACS Catal. 2018, 8, 6537–6551.
- 21Z. H. Lian, J. Wei, W. P. Shan, Y. B. Yu, P. M. Radjenovic, H. Zhang, G. Z. He, F. D. Liu, J. F. Li, Z. Q. Tian, H. He, J. Am. Chem. Soc. 2021, 143, 10454–10461.
- 22G. Z. He, Z. H. Lian, Y. B. Yu, Y. Yang, K. Liu, X. Y. Shi, Z. D. Yan, W. P. Shan, H. He, Sci. Adv. 2018, 4, eaau4637.
- 23R. J. G. Nuguid, D. Ferri, A. Marberger, M. Nachtegaal, O. Krocher, ACS Catal. 2019, 9, 6814–6820.
- 24J. Z. Hu, S. Xu, W.-Z. Li, M. Y. Hu, X. Deng, D. A. Dixon, M. Vasiliu, R. Craciun, Y. Wang, X. Bao, C. H. F. Peden, ACS Catal. 2015, 5, 3945–3952.
- 25Z. Lian, F. Liu, H. He, K. Liu, RSC Adv. 2015, 5, 37675–37681.
- 26L. Lietti, I. Nova, G. Ramis, L. Dall'Acqua, G. Busca, E. Giamello, P. Forzatti, F. Bregani, J. Catal. 1999, 187, 419–435.
- 27Z. Yan, W. Shan, X. Shi, G. He, Z. Lian, Y. Yu, Y. Shan, J. Liu, H. He, Catal. Today 2020, 355, 408–414.
- 28F. D. Liu, K. Asakura, H. He, W. P. Shan, X. Y. Shi, C. B. Zhang, Appl. Catal., B 2011, 103, 369–377.
- 29F. Giraud, J. Couble, C. Geantet, N. Guilhaume, S. Loridant, S. Gros, L. Porcheron, M. Kanniche, D. Bianchi, J. Phys. Chem. C 2018, 122, 24634–24651.
- 30A. Marberger, D. Ferri, M. Elsener, O. Krocher, Angew. Chem., Int. Ed. 2016, 55, 11989–11994.
- 31S. Xiong, J. Chen, H. Liu, X. Chen, W. Si, Z. Gong, Y. Peng, J. Li, Environ. Sci. Technol. 2022, 56, 3739–3747.
- 32M. Zhu, J.-K. Lai, U. Tumuluri, Z. Wu, I. E. Wachs, J. Am. Chem. Soc. 2017, 139, 15624–15627.
- 33D. R. Xu, W. Y. Qu, J. H. Liu, J. X. Chen, X. Fang, L. W. Chen, Z. Ma, X. Liu, X. F. Tang, Y. X. Chen, J. Mater. Chem. A 2023, 11, 24644–24650.
- 34T. Xu, K. C. Adamsen, H. Falsig, S. B. Rasmussen, Z. Li, S. Wendt, J. V. Lauritsen, Phys. Rev. Mater. 2020, 4, 130105.
- 35X. Fang, W. Y. Qu, T. Qin, X. L. Hu, L. W. Chen, Z. Ma, X. Liu, X. F. Tang, Environ. Sci. Technol. 2022, 56, 6631–6638.
- 36Y. Weijie, L. U. Hanfeng, Z. Bo, C. Yinfei, J. Mol. Catal. 2011, 25, 322–327.
- 37X. J. Liu, X. D. Gu, J. Y. Shen, Chin. J. Catal. 2003, 24, 674–680.
- 38S. Besselmann, C. Freitag, O. Hinrichsen, M. Muhler, Phys. Chem. Chem. Phys. 2001, 3, 4633–4638.
- 39C. Wang, S. Yang, H. Chang, Y. Peng, J. Li, Chem. Eng. J. 2013, 225, 520–527.
- 40X. Liu, X. Wu, T. Xu, D. Weng, Z. Si, R. Ran, Chin. J. Catal. 2016, 37, 1340–1346.
- 41M. I. Zaki, N. E. Fouad, S. A. A. Mansour, A. I. Muftah, Thermochim. Acta 2011, 523, 90–96.
- 42E. del Corro, M. Taravillo, V. G. Baonza, Phys. Rev. B 2012, 85, 033407.
- 43Y. Zhao, Y. Ding, W. Li, C. Liu, Y. Li, Z. Zhao, Y. Shan, F. Li, L. Sun, F. Li, Nat. Commun. 2023, 14, 4491.
- 44I. Song, J. Lee, G. Lee, J. W. Han, D. H. Kim, J. Phys. Chem. C 2018, 122, 16674–16682.
- 45G. He, M. Gao, Y. Peng, Y. Yu, W. Shan, H. He, Environ. Sci. Technol. 2021, 55, 6995–7003.
- 46A. Marberger, D. Ferri, M. Elsener, O. Kröcher, Angew. Chem., Int. Ed. 2016, 55, 11989–11994.