Cobalt-Catalyzed Enantioselective Hydrotrifluoromethoxylation of Aromatic Alkenes
Yafeng Si
State Key Laboratory and Institute of Elemento-Organic Chemistry, Frontiers Science Center for New Organic Matter, College of Chemistry, Nankai University, Tianjin, 300071 China
Search for more papers by this authorYuntao Liu
State Key Laboratory and Institute of Elemento-Organic Chemistry, Frontiers Science Center for New Organic Matter, College of Chemistry, Nankai University, Tianjin, 300071 China
Search for more papers by this authorFan Zhou
College of Chemistry and Environmental Science, Kashi University, Kashgar, 844008 China
Search for more papers by this authorLei Fang
State Key Laboratory and Institute of Elemento-Organic Chemistry, Frontiers Science Center for New Organic Matter, College of Chemistry, Nankai University, Tianjin, 300071 China
Search for more papers by this authorKang Wu
State Key Laboratory and Institute of Elemento-Organic Chemistry, Frontiers Science Center for New Organic Matter, College of Chemistry, Nankai University, Tianjin, 300071 China
Search for more papers by this authorCorresponding Author
Yu-Xin Luan
State Key Laboratory and Institute of Elemento-Organic Chemistry, Frontiers Science Center for New Organic Matter, College of Chemistry, Nankai University, Tianjin, 300071 China
E-mail: [email protected]; [email protected]
Search for more papers by this authorLi Chen
State Key Laboratory and Institute of Elemento-Organic Chemistry, Frontiers Science Center for New Organic Matter, College of Chemistry, Nankai University, Tianjin, 300071 China
Search for more papers by this authorCorresponding Author
Pingping Tang
State Key Laboratory and Institute of Elemento-Organic Chemistry, Frontiers Science Center for New Organic Matter, College of Chemistry, Nankai University, Tianjin, 300071 China
E-mail: [email protected]; [email protected]
Search for more papers by this authorYafeng Si
State Key Laboratory and Institute of Elemento-Organic Chemistry, Frontiers Science Center for New Organic Matter, College of Chemistry, Nankai University, Tianjin, 300071 China
Search for more papers by this authorYuntao Liu
State Key Laboratory and Institute of Elemento-Organic Chemistry, Frontiers Science Center for New Organic Matter, College of Chemistry, Nankai University, Tianjin, 300071 China
Search for more papers by this authorFan Zhou
College of Chemistry and Environmental Science, Kashi University, Kashgar, 844008 China
Search for more papers by this authorLei Fang
State Key Laboratory and Institute of Elemento-Organic Chemistry, Frontiers Science Center for New Organic Matter, College of Chemistry, Nankai University, Tianjin, 300071 China
Search for more papers by this authorKang Wu
State Key Laboratory and Institute of Elemento-Organic Chemistry, Frontiers Science Center for New Organic Matter, College of Chemistry, Nankai University, Tianjin, 300071 China
Search for more papers by this authorCorresponding Author
Yu-Xin Luan
State Key Laboratory and Institute of Elemento-Organic Chemistry, Frontiers Science Center for New Organic Matter, College of Chemistry, Nankai University, Tianjin, 300071 China
E-mail: [email protected]; [email protected]
Search for more papers by this authorLi Chen
State Key Laboratory and Institute of Elemento-Organic Chemistry, Frontiers Science Center for New Organic Matter, College of Chemistry, Nankai University, Tianjin, 300071 China
Search for more papers by this authorCorresponding Author
Pingping Tang
State Key Laboratory and Institute of Elemento-Organic Chemistry, Frontiers Science Center for New Organic Matter, College of Chemistry, Nankai University, Tianjin, 300071 China
E-mail: [email protected]; [email protected]
Search for more papers by this authorGraphical Abstract
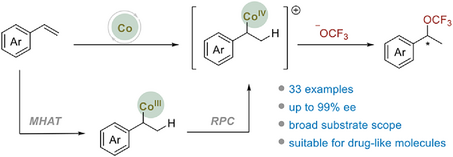
The synthesis of optically pure benzyl trifluoromethoxy (OCF3) compounds remains a challenging task. Here, we present the first enantioselective intermolecular hydrotrifluoromethoxylation reaction between aromatic alkenes and TFMS, catalyzed by chiral salen-Co complexes. This approach efficiently yields a variety of chiral benzylic trifluoromethoxy compounds with 75%–99% ee.
Abstract
The construction of optically pure benzyl trifluoromethoxy (OCF3) compounds continues to present challenges due to limited enantioselectivity-control or the necessity for OCF3-containing substrates in the only two previous reports. Herein, we have developed a salen-Co-catalyzed enantioselective hydrotrifluoromethoxylation reaction involving aromatic alkenes and trifluoromethyl arylsulfonate (TFMS). This method effectively produces a range of chiral benzyl trifluoromethoxy compounds with enantiomeric excesses ranging from 75% to 99%.
Conflict of Interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.
Supporting Information
| Filename | Description |
|---|---|
| anie202501680-sup-0001-SuppMat.pdf15.1 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1C. Hansch, A. Leo, R. W. Taft, Chem. Rev. 1991, 91, 165–195.
- 2J. Howard, R. Wall, Bull. Entomol. Res. 1995, 85, 71–77.
- 3D. Federsel, A. Herrmann, D. Christen, S. Sander, H. Willner, H. Oberhammer, J. Mol. Struct. 2001, 567-568, 127–136.
- 4J. Peter, B. Eckhard, R. L. Frederic, Mini. Rev. Med. Chem. 2007, 7, 1027–1034.
- 5C. Watson, D. R. Owen, D. Harding, K. Kon-I, M. L. Lewis, H. J. Mason, M. Matsumizu, T. Mukaiyama, M. Rodriguez-Lens, A. Shima, M. Takeuchi, I. Tran, T. Young, Bioorg. Med. Chem. Lett. 2011, 21, 4284–4287.
- 6L. A. Nguyen, H. He, C. Pham-Huy, Int. J. Biomed. Sci. 2006, 2, 85–100.
- 7S. Mitra, P. Chopra, Indian J Anaesth 2011, 55, 556.
- 8S.-J. Peng, Y.-Y. Zhu, C.-Y. Luo, P. Zhang, F.-Y. Wang, R.-X. Li, G.-Q. Lin, J.-G. Zhang, LMD 2024, 1, 100008.
- 9J.-H. Lin, Y.-L. Ji, J.-C. Xiao, Curr. Org. Chem. 2015, 19, 1541–1553.
- 10A. Tlili, F. Toulgoat, T. Billard, Angew. Chem. Int. Ed. 2016, 55, 11726–11735.
- 11K. N. Lee, J. W. Lee, M.-Y. Ngai, Tetrahedron 2018, 74, 7127–7135.
- 12B. Sahoo, M. N. Hopkinson, Angew. Chem. Int. Ed. 2018, 57, 7942–7944.
- 13J. W. Lee, K. N. Lee, M.-Y. Ngai, Angew. Chem. Int. Ed. 2019, 58, 11171–11181.
- 14X. Zhang, P. Tang, Sci. China Chem. 2019, 62, 525–532.
- 15X. Jiang, P. Tang, Chin. J. Chem. 2021, 39, 255–264.
- 16S. Barata-Vallejo, S. M. Bonesi, A. Postigo, Chem. - Eur. J. 2022, 28, e202201776.
- 17Y. Si, P. Tang, Chin. J. Chem. 2023, 41, 2179–2196.
- 18B.-Y. Hao, Y.-P. Han, Y. Zhang, Y.-M. Liang, Org. Biomol. Chem. 2023, 21, 4926–4954.
- 19M. B. Altaf, Y.-X. Luan, P. Tang, Sci. China Chem. accepted, https://doi.org/10.1007/s11426-024-2342-4.
- 20C. Huang, T. Liang, S. Harada, E. Lee, T. Ritter, J. Am. Chem. Soc. 2011, 133, 13308–13310.
- 21J.-B. Liu, C. Chen, L. Chu, Z.-H. Chen, X.-H. Xu, F.-L. Qing, Angew. Chem. Int. Ed. 2015, 54, 11839–11842.
- 22C. Chen, P. Chen, G. Liu, J. Am. Chem. Soc. 2015, 137, 15648–15651.
- 23X. Qi, P. Chen, G. Liu, Angew. Chem. Int. Ed. 2017, 56, 9517–9521.
- 24B. J. Jelier, P. F. Tripet, E. Pietrasiak, I. Franzoni, G. Jeschke, A. Togni, Angew. Chem. Int. Ed. 2018, 57, 13784–13789.
- 25W. Zheng, C. A. Morales-Rivera, J. W. Lee, P. Liu, M.-Y. Ngai, Angew. Chem. Int. Ed. 2018, 57, 9645–9649.
- 26M. Zhou, C. Ni, Y. Zeng, J. Hu, J. Am. Chem. Soc. 2018, 140, 6801–6805.
- 27J. Liu, Y. Wei, P. Tang, J. Am. Chem. Soc. 2018, 140, 15194–15199.
- 28S. Yang, M. Chen, P. Tang, Angew. Chem. Int. Ed. 2019, 58, 7840–7844.
- 29Z. Lu, T. Kumon, G. B. Hammond, T. Umemoto, Angew. Chem. Int. Ed. 2021, 60, 16171–16177.
- 30Y. Ouyang, X.-H. Xu, F.-L. Qing, Angew. Chem. Int. Ed. 2022, 61, e202114048.
- 31F. Toulgoat, T. Billard, Chem 2017, 2, 327–329.
- 32H. Kondo, M. Maeno, K. Hirano, N. Shibata, Chem. Commun. 2018, 54, 5522–5525.
- 33M. A. Hardy, H. Chachignon, D. Cahard, Asian J. Org. Chem. 2019, 8, 591–609.
- 34C. Chen, P. M. Pflüger, P. Chen, G. Liu, Angew. Chem. Int. Ed. 2019, 58, 2392–2396.
- 35Y. Liang, M. Maeno, Z. Zhao, N. Shibata, Molecules 2019, 24, 2774.
- 36X. Jiang, P. Tang, Chin. J. Chem. 2020, 38, 101–102.
- 37Y. Hou, Z. Zhang, X. Sun, Z. Yang, Y.-X. Luan, P. Tang, Angew. Chem. Int. Ed. 2023, 62, e202218919.
- 38L. Wang, Y. Si, Y.-X. Luan, P. Tang, CCS Chem. 2024, 6, 1885–1894.
- 39Y. S. K. Dota, Y. Akioka, N. Maehata, S. Arimori, WO2018097318 A1, 2018.
- 40J. J. Braanalt, Maria; Nordqvist, Anneli; O'Mahony, Gavin; Swanson, Marianne, US20230406840 A1, 2023.
- 41O. Marrec, T. Billard, J.-P. Vors, S. Pazenok, B. R. Langlois, Adv. Synth. Catal. 2010, 352, 2831–2837.
- 42X. Jiang, Z. Deng, P. Tang, Angew. Chem. Int. Ed. 2018, 57, 292–295.
- 43H. Yang, F. Wang, X. Jiang, Y. Zhou, X. Xu, P. Tang, Angew. Chem. Int. Ed. 2018, 57, 13266–13270.
- 44J. Yu, J.-H. Lin, D. Yu, R. Du, J.-C. Xiao, Nat. Commun. 2019, 10, 5362.
- 45Y. Li, Y. Yang, J. Xin, P. Tang, Nat. Commun. 2020, 11, 755.
- 46J. J. Newton, B. J. Jelier, M. Meanwell, R. E. Martin, R. Britton, C. M. Friesen, Org. Lett. 2020, 22, 1785–1790.
- 47G. Duran-Camacho, D. M. Ferguson, J. W. Kampf, D. C. Bland, M. S. Sanford, Org. Lett. 2021, 23, 5138–5142.
- 48C. Bonnefoy, E. Chefdeville, A. Panosian, G. Hanquet, F. R. Leroux, F. Toulgoat, T. Billard, Chem. - Eur. J. 2021, 27, 15986–15991.
- 49C. Bonnefoy, A. Panossian, G. Hanquet, F. R. Leroux, F. Toulgoat, T. Billard, Chem. - Eur. J. 2023, 29, e202301513.
- 50W.-J. Yuan, C.-L. Tong, X.-H. Xu, F.-L. Qing, J. Org. Chem. 2023, 88, 4434–4441.
- 51Z. Deng, L. Meng, X. Bing, S. Niu, X. Zhang, J. Peng, Y.-X. Luan, L. Chen, P. Tang, J. Am. Chem. Soc. 2024, 146, 2325–2332.
- 52M. Spennacchio, M. Bernús, J. Stanić, D. Mazzarella, M. Colella, J. J. Douglas, O. Boutureira, T. Noël, Science 2024, 385, 991–996.
- 53S. Guo, F. Cong, R. Guo, L. Wang, P. Tang, Nat. Chem. 2017, 9, 546–551.
- 54W. Huang, X. Wan, Q. Shen, Angew. Chem. Int. Ed. 2017, 56, 11986–11989.
- 55X. Shen, X. Chen, J. Chen, Y. Sun, Z. Cheng, Z. Lu, Nat. Commun. 2020, 11, 783.
- 56G. Zhang, Q. Zhang, Chem Catal. 2023, 3, 100526.
- 57S. N. Anderson, D. H. Ballard, J. Z. Chrzastowski, D. Dodd, M. D. Johnson, J. Chem. Soc., Chem. Commun. 1972, 685–686.
- 58R. H. Magnuson, J. Halpern, I. Y. Levitin, M. E. Vol'pin, J. Chem. Soc., Chem. Commun. 1978, 44–46.
- 59E. E. Touney, N. J. Foy, S. V. Pronin, J. Am. Chem. Soc. 2018, 140, 16982–16987.
- 60T. Nagai, N. Mimata, Y. Terada, C. Sebe, H. Shigehisa, Org. Lett. 2020, 22, 5522–5527.
- 61S. Ohuchi, H. Koyama, H. Shigehisa, ACS Catal. 2021, 11, 900–906.
- 62C. A. Discolo, E. E. Touney, S. V. Pronin, J. Am. Chem. Soc. 2019, 141, 17527–17532.
- 63K. Ebisawa, K. Izumi, Y. Ooka, H. Kato, S. Kanazawa, S. Komatsu, E. Nishi, H. Shigehisa, J. Am. Chem. Soc. 2020, 142, 13481–13490.
- 64T. Qin, G. Lv, Q. Meng, G. Zhang, T. Xiong, Q. Zhang, Angew. Chem. Int. Ed. 2021, 60, 25949–25957.
- 65T. Qin, G. Lv, H. Miao, M. Guan, C. Xu, G. Zhang, T. Xiong, Q. Zhang, Angew. Chem. Int. Ed. 2022, 61, e202201967.
- 66H. Miao, M. Guan, T. Xiong, G. Zhang, Q. Zhang, Angew. Chem. Int. Ed. 2023, 62, e202213913.
- 67M. Guan, L. Zhu, Y. Wang, G. Zhang, H. Miao, B. Chen, Q. Zhang, Chem Catal. 2024, 4, 101126.
- 68Q. Meng, T. Qin, H. Miao, G. Zhang, Q. Zhang, Sci. China Chem. 2024, 67, 2002–2008.
- 69M. Guan, Y. Wang, T. Yin, Y. Gao, H. Miao, G. Zhang, T. Xiong, Q. Zhang, CCS Chem. 2025, 7, 765–775.
- 70P. G. Kalomenopoulos, B. Emayavaramban, C. P. Johnston, Angew. Chem. Int. Ed. 2025, 64, e202414342.
- 71S. Xu, Y. Shao, H. Zheng, X. Leng, X.-S. Xue, Q. Shen, New J. Chem. 2022, 46, 20760–20767.
- 72S. H. Park, J. Jang, K. Shin, H. Kim, ACS Catal. 2022, 12, 10572–10580.
- 73X.-L. Zhou, F. Yang, H.-L. Sun, Y.-N. Yin, W.-T. Ye, R. Zhu, J. Am. Chem. Soc. 2019, 141, 7250–7255.
- 74H. Shigehisa, T. Aoki, S. Yamaguchi, N. Shimizu, K. Hiroya, J. Am. Chem. Soc. 2013, 135, 10306–10309.
- 75H. Shigehisa, N. Koseki, N. Shimizu, M. Fujisawa, M. Niitsu, K. Hiroya, J. Am. Chem. Soc. 2014, 136, 13534–13537.
- 76H. Shigehisa, M. Hayashi, H. Ohkawa, T. Suzuki, H. Okayasu, M. Mukai, A. Yamazaki, R. Kawai, H. Kikuchi, Y. Satoh, A. Fukuyama, K. Hiroya, J. Am. Chem. Soc. 2016, 138, 10597–10604.
- 77S. Date, K. Hamasaki, K. Sunagawa, H. Koyama, C. Sebe, K. Hiroya, H. Shigehisa, ACS Catal. 2020, 10, 2039–2045.
- 78R. L. Sweany, J. Halpern, J. Am. Chem. Soc. 1977, 99, 8335–8337.
- 79J. L. Male, B. E. Lindfors, K. J. Covert, D. R. Tyler, J. Am. Chem. Soc. 1998, 120, 13176–13186.
- 80C. V. Wilson, D. Kim, A. Sharma, R. X. Hooper, R. Poli, B. M. Hoffman, P. L. Holland, J. Am. Chem. Soc. 2022, 144, 10361–10367.
- 81S. S. Lande, J. K. Kochi, J. Am. Chem. Soc. 1968, 90, 5196–5207.
- 82J. Halpern, M. S. Chan, J. Hanson, T. S. Roche, J. A. Topich, J. Am. Chem. Soc. 1975, 97, 1606–1608.
- 83J. Topich, J. Halpern, Inorg. Chem. 1979, 18, 1339–1343.
- 84M. E. Vol'pin, I. Y. Levitin, A. L. Sigan, J. Halpern, G. M. Tom, Inorg. Chim. Acta 1980, 41, 271–277.