Electronic Structure Origins of Distinct Hydrogenation Activities Observed for Linear and Bent Bimetallic μ-Nitrides
Mengdi Huang
State Key Laboratory of Catalysis, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian, 116023 P.R. China
University of Chinese Academy of Sciences, Beijing, 100049 P.R. China
These authors contributed equally to this work.
Search for more papers by this authorLuyang Sun
State Key Laboratory of Fine Chemicals, Frontier Science Center for Smart Materials, Dalian University of Technology, Dalian, 116024 P.R. China
These authors contributed equally to this work.
Search for more papers by this authorZihe Song
State Key Laboratory of Fine Chemicals, Frontier Science Center for Smart Materials, Dalian University of Technology, Dalian, 116024 P.R. China
These authors contributed equally to this work.
Search for more papers by this authorHaowei Chen
State Key Laboratory of Catalysis, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian, 116023 P.R. China
Search for more papers by this authorDr. Pan Gao
State Key Laboratory of Catalysis, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian, 116023 P.R. China
Search for more papers by this authorProf. Guangjin Hou
State Key Laboratory of Catalysis, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian, 116023 P.R. China
Search for more papers by this authorDr. Georgi L. Stoychev
Max-Planck-Institut für Kohlenforschung, Kaiser-Wilhelmplatz 1, D-45470 Mülheim an der Ruhr, Germany
Search for more papers by this authorProf. Baomin Wang
State Key Laboratory of Fine Chemicals, Frontier Science Center for Smart Materials, Dalian University of Technology, Dalian, 116024 P.R. China
Search for more papers by this authorCorresponding Author
Dr. Dawei Yang
State Key Laboratory of Fine Chemicals, Frontier Science Center for Smart Materials, Dalian University of Technology, Dalian, 116024 P.R. China
Email: [email protected], [email protected], [email protected]
Search for more papers by this authorCorresponding Author
Prof. Jingping Qu
State Key Laboratory of Fine Chemicals, Frontier Science Center for Smart Materials, Dalian University of Technology, Dalian, 116024 P.R. China
State Key Laboratory of Bioreactor Engineering, Collaborative Innovation Centre for Biomanufacturing, Frontiers Science Center for Materiobiology and Dynamic Chemistry, East China University of Science and Technology, Shanghai, 200237 P.R. China
Email: [email protected], [email protected], [email protected]
Search for more papers by this authorCorresponding Author
Prof. Shengfa Ye
State Key Laboratory of Catalysis, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian, 116023 P.R. China
Key Laboratory of Bioinorganic and Synthetic Chemistry of Ministry of Education, Guangdong Basic Research Center of Excellence for Functional Molecular Engineering, School of Chemistry, IGCME, Sun Yat-sen University, Guangzhou, 510275 P.R. China
Email: [email protected], [email protected], [email protected]
Search for more papers by this authorMengdi Huang
State Key Laboratory of Catalysis, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian, 116023 P.R. China
University of Chinese Academy of Sciences, Beijing, 100049 P.R. China
These authors contributed equally to this work.
Search for more papers by this authorLuyang Sun
State Key Laboratory of Fine Chemicals, Frontier Science Center for Smart Materials, Dalian University of Technology, Dalian, 116024 P.R. China
These authors contributed equally to this work.
Search for more papers by this authorZihe Song
State Key Laboratory of Fine Chemicals, Frontier Science Center for Smart Materials, Dalian University of Technology, Dalian, 116024 P.R. China
These authors contributed equally to this work.
Search for more papers by this authorHaowei Chen
State Key Laboratory of Catalysis, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian, 116023 P.R. China
Search for more papers by this authorDr. Pan Gao
State Key Laboratory of Catalysis, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian, 116023 P.R. China
Search for more papers by this authorProf. Guangjin Hou
State Key Laboratory of Catalysis, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian, 116023 P.R. China
Search for more papers by this authorDr. Georgi L. Stoychev
Max-Planck-Institut für Kohlenforschung, Kaiser-Wilhelmplatz 1, D-45470 Mülheim an der Ruhr, Germany
Search for more papers by this authorProf. Baomin Wang
State Key Laboratory of Fine Chemicals, Frontier Science Center for Smart Materials, Dalian University of Technology, Dalian, 116024 P.R. China
Search for more papers by this authorCorresponding Author
Dr. Dawei Yang
State Key Laboratory of Fine Chemicals, Frontier Science Center for Smart Materials, Dalian University of Technology, Dalian, 116024 P.R. China
Email: [email protected], [email protected], [email protected]
Search for more papers by this authorCorresponding Author
Prof. Jingping Qu
State Key Laboratory of Fine Chemicals, Frontier Science Center for Smart Materials, Dalian University of Technology, Dalian, 116024 P.R. China
State Key Laboratory of Bioreactor Engineering, Collaborative Innovation Centre for Biomanufacturing, Frontiers Science Center for Materiobiology and Dynamic Chemistry, East China University of Science and Technology, Shanghai, 200237 P.R. China
Email: [email protected], [email protected], [email protected]
Search for more papers by this authorCorresponding Author
Prof. Shengfa Ye
State Key Laboratory of Catalysis, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian, 116023 P.R. China
Key Laboratory of Bioinorganic and Synthetic Chemistry of Ministry of Education, Guangdong Basic Research Center of Excellence for Functional Molecular Engineering, School of Chemistry, IGCME, Sun Yat-sen University, Guangzhou, 510275 P.R. China
Email: [email protected], [email protected], [email protected]
Search for more papers by this authorGraphical Abstract
Abstract
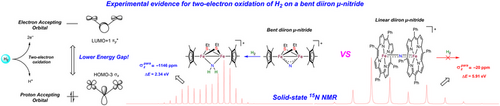
Hydrogenation of metal nitrides is of particular interest due to the direct relevance to Haber–Bosch ammonia synthesis. Notably, for all bi- and multi-nuclear bridging nitrides reported thus far, only those featuring bent M─N─M cores can react with dihydrogen (H2) and related H2-derived species, while the vast majority of linear M─N─M congeners cannot. Herein, we present a detailed electronic-structure study of prototypical bimetallic bent μ-nitrides [Cp*FeIV(μ-SEt)2(μ-N)FeIVCp*][PF6] (1, Cp* = η5-C5Me5) and [Cp*CoIII(μ-SAd)(μ-N)CoIIICp*] (3, Ad = adamantyl) and linear μ-nitride [(TPP)FeIV(μ-N)FeIV(TPP)][PF6] (2, TPP2− = 5,10,15,20-tetraphenylporphinato), as well as μ-imide [Cp*CoIII(μ-SAd)(μ-NH)CoIIICp*][BPh4] (4), using various spectroscopic techniques, in particular, 15N solid-state nuclear magnetic resonance, coupled with density functional theory calculations. An in-depth analysis of their distinct 15N shielding tensors revealed that bent μ-nitrides invariably possess a high-lying proton-accepting molecular orbital (MO) and a low-lying electron-accepting MO. These electronic-structure features are key to the bent μ-nitrides affecting hydrogenolysis via either two-electron oxidation of H2 or H2 heterolysis. However, because of symmetry, linear μ-nitrides lack potent proton-accepting MOs, which rationalizes their disparate hydrogenation activities.
Conflict of Interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the Supporting Information of this article.
Supporting Information
| Filename | Description |
|---|---|
| anie202424571-sup-0001-SuppMat.pdf11.5 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1J. Hohenberger, K. Ray, K. Meyer, Nat. Commun. 2012, 3, 720.
- 2T. M. Buscagan, D. C. Rees, Joule 2019, 3, 2662–2678.
- 3A. J. Jasniewski, C. C. Lee, M. W. Ribbe, Y. Hu, Chem. Rev. 2020, 120, 5107–5157.
- 4L. C. Seefeldt, Z.-Y. Yang, D. A. Lukoyanov, D. F. Harris, D. R. Dean, S. Raugei, B. M. Hoffman, Chem. Rev. 2020, 120, 5082–5106.
- 5R. Schlögl, Angew. Chem. Int. Ed. 2003, 42, 2004–2008.
- 6G. Ertl, Angew. Chem. Int. Ed. 2008, 47, 3524–3535.
- 7H. Liu, Chin. J. Catal. 2014, 35, 1619–1640.
- 8J. F. Berry, Comments Inorg. Chem. 2009, 30, 28–66.
- 9J. M. Smith, in Progress in Organic Chemistry, Vol. 58, John Wiley & Sons, Inc., Hoboken, NJ 2014, pp. 417–470.
- 10Z.-J. Lv, J. Wei, W.-X. Zhang, P. Chen, D. Deng, Z.-J. Shi, Z. Xi, Natl. Sci. Rev. 2020, 7, 1564–1583.
- 11S. Kim, F. Loose, P. J. Chirik, Chem. Rev. 2020, 120, 5637–5681.
- 12S. J. K. Forrest, B. Schluschaß, E. Y. Yuzik-Klimova, S. Schneider, Chem. Rev. 2021, 121, 6522–6587.
- 13Q. Zhuo, X. Zhou, T. Shima, Z. Hou, Angew. Chem. Int. Ed. 2023, 62, e202218606.
- 14M. N. Cosio, D. C. Powers, Nat. Rev. Chem. 2023, 7, 424–438.
- 15S. D. Brown, M. P. Mehn, J. C. Peters, J. Am. Chem. Soc. 2005, 127, 13146–13147.
- 16T. Shima, S. Hu, G. Luo, X. Kang, Y. Luo, Z. Hou, Science 2013, 340, 1549–1552.
- 17M. Reiners, D. Baabe, K. Münster, M. K. Zaretzke, M. Freytag, P. G. Jones, Y. Coppel, S. Bontemps, I. D. Rosal, L. Maron, M. D. Walter, Nat. Chem. 2020, 12, 740–746.
- 18D. Sengupta, C. Sandoval-Pauker, E. Schueller, A. M. Encerrado-Manriquez, A. Metta-Magaña, W.-Y. Lee, R. Seshadri, B. Pinter, S. Fortier, J. Am. Chem. Soc. 2020, 142, 8233–8242.
- 19J. Schöffel, A. Y. Rogachev, S. DeBeer George, P. Burger, Angew. Chem. Int. Ed. 2009, 48, 4734–4738.
- 20L. Chatelain, E. Louyriac, I. Douair, E. Lu, F. Tuna, A. J. Wooles, B. M. Gardner, L. Maron, S. T. Liddle, Nat. Commun. 2020, 11, 337.
- 21T. Mei, P. Zhang, Z. Song, B. Wang, J. Qu, S. Ye, D. Yang, J. Am. Chem. Soc. 2023, 145, 20578–20587.
- 22B. Askevold, J. T. Nieto, S. Tussupbayev, M. Diefenbach, E. Herdtweck, M. C. Holthausen, S. Schneider, Nat. Chem. 2011, 3, 532–537.
- 23F. S. Schendzielorz, M. Finger, C. Volkmann, C. Würtele, S. Schneider, Angew. Chem. Int. Ed. 2016, 55, 11417–11420.
- 24M. Falcone, L. Chatelain, R. Scopelliti, I. Živković, M. Mazzanti, Nature 2017, 547, 332–335.
- 25S. Kim, H. Zhong, Y. Park, F. Loose, P. J. Chirik, J. Am. Chem. Soc. 2020, 142, 9518–9524.
- 26Y. Zhang, J. Zhao, D. Yang, B. Wang, Y. Zhou, J. Wang, H. Chen, T. Mei, S. Ye, J. Qu, Nat. Chem. 2022, 14, 46–52.
- 27J. F. Berry, E. Bill, E. Bothe, S. D. George, B. Mienert, F. Neese, K. Wieghardt, Science 2006, 312, 1937–1941.
- 28J. J. Scepaniak, J. A. Young, R. P. Bontchev, J. M. Smith, Angew. Chem. Int. Ed. 2009, 48, 3158–3160.
- 29J. J. Scepaniak, C. S. Vogel, M. M. Khusniyarov, F. W. Heinemann, K. Meyer, J. M. Smith, Science 2011, 331, 1049–1052.
- 30H.-C. Chang, B. Mondal, H. Fang, F. Neese, E. Bill, S. Ye, J. Am. Chem. Soc. 2019, 141, 2421–2434.
- 31S. D. Brown, J. C. Peters, J. Am. Chem. Soc. 2005, 127, 1913–1923.
- 32M. Reiners, M. Maekawa, C. G. Daniliuc, M. Freytag, P. G. Jones, P. S. White, J. Hohenberger, J. Sutter, K. Meyer, L. Maron, M. D. Walter, Chem. Sci. 2017, 8, 4108–4122.
- 33M. V. Bennett, S. Stoian, E. L. Bominaar, E. Münck, R. H. Holm, J. Am. Chem. Soc. 2005, 127, 12378–12386.
- 34M. M. Rodriguez, E. Bill, W. W. Brennessel, P. L. Holland, Science 2011, 334, 780–783.
- 35T. M. Powers, T. A. Betley, J. Am. Chem. Soc. 2013, 135, 12289–12296.
- 36D. M. Ermert, J. B. Gordon, K. A. Abboud, L. J. Murray, Inorg. Chem. 2015, 54, 9282–9289.
- 37M. J. Drance, C. C. Mokhtarzadeh, M. Melaimi, D. W. Agnew, C. E. Moore, A. L. Rheingold, J. S. Figueroa, Angew. Chem. Int. Ed. 2018, 57, 13057–13061.
- 38G. Xu, J. Zhou, Z. Wang, R. H. Holm, X.-D. Chen, Angew. Chem. Int. Ed. 2019, 58, 16469–16473.
- 39L. N. V. Le, G. A. Bailey, A. G. Scott, T. Agapie, Proc. Natl. Acad. Sci. USA 2021, 118, e2109241118.
- 40Representative linear diiron μ-nitrides: D. A. Summerville, I. A. Cohen, J. Am. Chem. Soc. 1976, 98, 1747–1752.
- 41L. A. Bottomley, J. N. Gorce, V. L. Goedken, C. Ercolani, Inorg. Chem. 1985, 24, 3733–3737.
- 42T. Jüstel, T. Weyhermüller, K. Wieghardt, E. Bill, M. Lengen, A. X. Trautwein, P. Hildebrandt, Angew. Chem. Int. Ed. 1995, 34, 669–672.
- 43E. V. Kudrik, P. Afanasiev, L. X. Alvarez, P. Dubourdeaux, M. Clémancey, J. M. Latour, G. Blondin, D. Bouchu, F. Albrieux, S. E. Nefedov, A. B. Sorokin, Nat. Chem. 2012, 4, 1024–1029.
- 44S. Zhang, P. Cui, T. Liu, Q. Wang, T. J. Longo, L. M. Thierer, B. C. Manor, M. R. Gau, P. J. Carroll, G. C. Papaefthymiou, N. C. Tomson, Angew. Chem. Int. Ed. 2020, 59, 15215–15219.
- 45C. Cordes, I. Klawitter, I. Rüter, S. Dechert, S. Demeshko, S. Ye, F. Meyer, Inorg. Chem. 2022, 61, 7153–7164.
- 46D. M. King, F. Tuna, E. J. L. McInnes, J. McMaster, W. Lewis, A. J. Blake, S. T. Liddle, Science 2012, 337, 717–720.
- 47P. Cui, Q. Wang, S. P. McCollom, B. C. Manor, P. J. Carroll, N. C. Tomson, Angew. Chem. Int. Ed. 2017, 56, 15979–15983.
- 48J. A. Buss, C. Cheng, T. Agapie, Angew. Chem. Int. Ed. 2018, 57, 9670–9674.
- 49H. Shi, R. Liang, D. L. Phillips, H. K. Lee, W. L. Man, K.-C. Lau, S. M. Yiu, T.-C. Lau, J. Am. Chem. Soc. 2022, 144, 7588–7593.
- 50M. G. Jafari, D. Fehn, A. Reinholdt, C. Hernández-Prieto, P. Patel, M. R. Gau, P. J. Carroll, J. Krzystek, C. Liu, A. Ozarowski, J. Telser, M. Delferro, K. Meyer, D. J. Mindiola, J. Am. Chem. Soc. 2022, 144, 10201–10219.
- 51Ü. İşci, P. Afanasiev, J.-M. M. Millet, E. V. Kudrik, V. Ahsen, A. B. Sorokin, Dalton Trans. 2009, 7410–7420.
- 52T. Jüstel, M. Müller, T. Weyhermüller, C. Kressl, E. Bill, P. Hildebrandt, M. Lengen, M. Grodzicki, A. X. Trautwein, B. Nuber, K. Wieghardt, Chem. - Eur. J. 1999, 5, 793–810.
- 53C. Ercolani, J. Jubb, G. Pennesi, U. Russo, G. Trigiante, Inorg. Chem. 1995, 34, 2535–2541.
- 54S. K. Dutta, U. Beckmann, E. Bill, T. Weyhermüller, K. Wieghardt, Inorg. Chem. 2000, 39, 3355–3364.
- 55D. R. English, D. N. Hendrickson, K. S. Suslick, Inorg. Chem. 1983, 22, 367–368.
- 56C. Ercolani, M. Gardini, G. Pennesi, G. Rossi, U. Russo, Inorg. Chem. 1988, 27, 422–424.
- 57S. Ye, E. Bill, F. Neese, Inorg. Chem. 2016, 55, 3468–3474.
- 58E. L. Sceats, J. S. Figueroa, C. C. Cummins, N. M. Loening, P. Van der Wel, R. G. Griffin, Polyhedron 2004, 23, 2751–2768.
- 59J. Du, J. A. Seed, V. E. J. Berryman, N. Kaltsoyannis, R. W. Adams, D. Lee, S. T. Liddle, Nat. Commun. 2021, 12, 5649.
- 60J. B. Greco, J. C. Peters, T. A. Baker, W. M. Davis, C. C. Cummins, G. Wu, J. Am. Chem. Soc. 2001, 123, 5003–5013.
- 61F. Blanc, J.-M. Basset, C. Copéret, A. Sinha, Z. J. Tonzetich, R. R. Schrock, X. Solans-Monfort, E. Clot, O. Eisenstein, A. Lesage, L. Emsley, J. Am. Chem. Soc. 2008, 130, 5886–5900.
- 62D. P. Estes, C. P. Gordon, A. Fedorov, W.-C. Liao, H. Ehrhorn, C. Bittner, M. L. Zier, D. Bockfeld, K. W. Chan, O. Eisenstein, C. Raynaud, M. Tamm, C. Copéret, J. Am. Chem. Soc. 2017, 139, 17597–17607.
- 63C. P. Gordon, S. Shirase, K. Yamamoto, R. A. Andersen, O. Eisenstein, C. Copéret, Proc. Natl. Acad. Sci. USA 2018, 115, E5867–E5876.
- 64L. Foppa, K. Yamamoto, W.-C. Liao, A. Comas-Vives, C. Copéret, J. Phys. Chem. Lett. 2018, 9, 3348–3353.
- 65C. P. Gordon, D. B. Culver, M. P. Conley, O. Eisenstein, R. A. Andersen, C. Copéret, J. Am. Chem. Soc. 2019, 141, 648–656.
- 66C. P. Gordon, C. Raynaud, R. A. Andersen, C. Copéret, O. Eisenstein, Acc. Chem. Res. 2019, 52, 2278–2289.
- 67C. F. Baker, J. A. Seed, R. W. Adams, D. Lee, S. T. Liddle, Chem. Sci. 2024, 15, 238–249.
- 68G. Wu, D. Rovnyak, M. J. A. Johnson, N. C. Zanetti, D. G. Musaev, K. Morokuma, R. R. Schrock, R. G. Griffin, C. C. Cummins, J. Am. Chem. Soc. 1996, 118, 10654–10655.
- 69W. J. Transue, J. Yang, M. Nava, I. V. Sergeyev, T. J. Barnum, M. C. McCarthy, C. C. Cummins, J. Am. Chem. Soc. 2018, 140, 17985–17991.
- 70J. Du, J. Hurd, J. A. Seed, G. Balázs, M. Scheer, R. W. Adams, D. Lee, S. T. Liddle, J. Am. Chem. Soc. 2023, 145, 21766–21784.
- 71F. Neese, WIREs Comput. Mol. Sci. 2022, 12, 1606.
- 72G. L. Stoychev, A. A. Auer, R. Izsák, F. Neese, J. Chem. Theory Comput. 2018, 14, 619–637.
- 73A. D. Becke, Phys. Rev. A 1988, 38, 3098–3100.
- 74J. P. Perdew, Phys. Rev. B 1986, 33, 8822–8824.
- 75J. P. Perdew, K. Burke, M. Ernzerhof, Phys. Rev. Lett. 1996, 77, 3865–3868.
- 76C. Adamo, V. Barone, J. Chem. Phys. 1999, 110, 6158–6170.
- 77C. Lee, W. Yang, R. G. Parr, Phys. Rev. B 1988, 37, 785–789.
- 78A. D. Becke, J. Chem. Phys. 1993, 98, 5648–5652.
- 79S. Ye, C. Y. Geng, S. Shaik, F. Neese, Phys. Chem. Chem. Phys. 2013, 15, 8017.
- 80A. Z. Spentzos, N. C. Tomson, Inorg. Chem. 2021, 60, 6889–6899.
- 81J. Mason, Solid State Nucl. Magn. Reson. 1993, 2, 285–288.
- 82R. K. Harris, E. D. Becker, S. M. Cabral de Menezes, R. Goodfellow, P. Granger, Pure Appl. Chem. 2001, 73, 1795–1818.
- 83P. Pyykkö, Theor. Chem. Acc. 2000, 103, 214–216.
- 84N. F. Ramsey, Phys. Rev. 1950, 78, 699–703.
- 85N. F. Ramsey, Phys. Rev. 1952, 86, 243–246.
- 86J. Mason, Multinuclear NMR, Springer Science & Business Media, Berlin 1987, pp. 335–367.
10.1007/978-1-4613-1783-8_12 Google Scholar
- 87C. M. Widdifield, R. W. Schurko, Magn. Reson. A 2009, 34A, 91–123.
- 88A. J. Pell, G. Pintacuda, C. P. Grey, Prog. Nucl. Magn. Reson. Spectrosc. 2019, 111, 1–271.
- 89A. Buckingham, P. Stephens, J. Chem. Soc. 1964, 2747.
- 90A. Buckingham, S. Malm, Mol. Phys. 1971, 22, 1127–1130.
- 91Y. Kakiuchi, P. S. Karmakar, J. Roudin, I. A. Tonks, C. Copéret, J. Am. Chem. Soc. 2024, 146, 9860–9870.
- 92L. A. Bottomley, B. B. Garrett, Inorg. Chem. 1982, 21, 1260–1263.