Silylchromium-Catalyzed Selective Semihydrogenation of Undirected Allenes by Sila-Metalation and β-Silyl Elimination
Zheng Luo
Key Laboratory of Green Chemistry & Technology, Ministry of Education, College of Chemistry, Sichuan University, Chengdu, 610064 China
Both authors contributed equally to this work.
Search for more papers by this authorLinhong Long
Beijing National Laboratory for Molecular Sciences (BNLMS), Key Laboratory of Photochemistry, Institute of Chemistry, Chinese Academy of Sciences, Chinese Academy of Sciences, Beijing, 100190 China
Both authors contributed equally to this work.
Search for more papers by this authorShuaiyong Zhao
Key Laboratory of Green Chemistry & Technology, Ministry of Education, College of Chemistry, Sichuan University, Chengdu, 610064 China
Search for more papers by this authorXiaoyu Zhang
Key Laboratory of Green Chemistry & Technology, Ministry of Education, College of Chemistry, Sichuan University, Chengdu, 610064 China
Search for more papers by this authorYong Peng
Key Laboratory of Green Chemistry & Technology, Ministry of Education, College of Chemistry, Sichuan University, Chengdu, 610064 China
Search for more papers by this authorProf. Dr. Meiming Luo
Key Laboratory of Green Chemistry & Technology, Ministry of Education, College of Chemistry, Sichuan University, Chengdu, 610064 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Hui Chen
Beijing National Laboratory for Molecular Sciences (BNLMS), Key Laboratory of Photochemistry, Institute of Chemistry, Chinese Academy of Sciences, Chinese Academy of Sciences, Beijing, 100190 China
E-mail: [email protected]; [email protected]
Search for more papers by this authorCorresponding Author
Prof. Dr. Xiaoming Zeng
Key Laboratory of Green Chemistry & Technology, Ministry of Education, College of Chemistry, Sichuan University, Chengdu, 610064 China
E-mail: [email protected]; [email protected]
Search for more papers by this authorZheng Luo
Key Laboratory of Green Chemistry & Technology, Ministry of Education, College of Chemistry, Sichuan University, Chengdu, 610064 China
Both authors contributed equally to this work.
Search for more papers by this authorLinhong Long
Beijing National Laboratory for Molecular Sciences (BNLMS), Key Laboratory of Photochemistry, Institute of Chemistry, Chinese Academy of Sciences, Chinese Academy of Sciences, Beijing, 100190 China
Both authors contributed equally to this work.
Search for more papers by this authorShuaiyong Zhao
Key Laboratory of Green Chemistry & Technology, Ministry of Education, College of Chemistry, Sichuan University, Chengdu, 610064 China
Search for more papers by this authorXiaoyu Zhang
Key Laboratory of Green Chemistry & Technology, Ministry of Education, College of Chemistry, Sichuan University, Chengdu, 610064 China
Search for more papers by this authorYong Peng
Key Laboratory of Green Chemistry & Technology, Ministry of Education, College of Chemistry, Sichuan University, Chengdu, 610064 China
Search for more papers by this authorProf. Dr. Meiming Luo
Key Laboratory of Green Chemistry & Technology, Ministry of Education, College of Chemistry, Sichuan University, Chengdu, 610064 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Hui Chen
Beijing National Laboratory for Molecular Sciences (BNLMS), Key Laboratory of Photochemistry, Institute of Chemistry, Chinese Academy of Sciences, Chinese Academy of Sciences, Beijing, 100190 China
E-mail: [email protected]; [email protected]
Search for more papers by this authorCorresponding Author
Prof. Dr. Xiaoming Zeng
Key Laboratory of Green Chemistry & Technology, Ministry of Education, College of Chemistry, Sichuan University, Chengdu, 610064 China
E-mail: [email protected]; [email protected]
Search for more papers by this authorGraphical Abstract
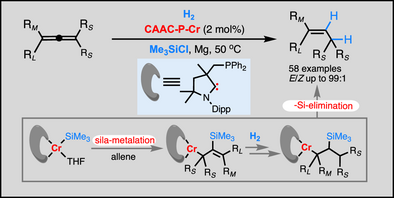
Substituted allenes without a directing group underwent chromium-catalyzed chemo-, stereo-, and regioselective semihydrogenation with an (alkyl)(amino)carbene-phosphine ligand and a chlorosilane additive. The silylchromium complex formed in situ promotes semihydrogenation through a pathway involving sila-metalation of the allene and β-silyl elimination, with the silyl group acting as a noninnocent ligand to give E-alkene products selectively.
Abstract
The semihydrogenation of simple allenes remains an issue because of the difficulty in controlling of selectivity without coordination assistance from directing groups, wherein over-hydrogenated alkanes and E/Z-mixed stereo- and regio-isomers can be potentially formed. We report here an electron-rich cyclic (alkyl)(amino)carbene (CAAC)-phosphine-ligated chromium(III) complex serving as precatalyst in promoting the selective semihydrogenation of undirected allenes by forming active silylchromium species. The semihydrogenation allows the selective addition of H2 to the less substituted and electron-rich alkyl-substituted C═C bonds of allenes while enabling the suppression of competitive over-hydrogenation, proving generally to form trisubstituted E-alkenes in high chemo-, regio-, and stereoselectivity. By this CAAC-phosphine-Cr-catalyzed selective semihydrogenation, unsaturated nitro, nitrile, alkenyl, and hydrogenation-sensitive bromide, chloride, and fluoride were compatible with various substitution environments. The in situ forming silylchromium as active catalyst by reaction of low-valent chromium complex with chlorosilane was established by studying the catalytic and stoichiometric reactivity of this species in the semihydrogenation. Deuterium labeling experiments, HRMS, XPS, EPR, and magnetic susceptibility analysis of the related intermediates and theoretical studies support a pathway involving sila-metalation of allene and β-silyl elimination processes with silyl as noninnocent ligand, leading to the E-alkene products.
Conflict of Interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the Supporting Information of this article.
Supporting Information
| Filename | Description |
|---|---|
| anie202424273-sup-0001-SuppMat.pdf16.1 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1S. Ma, Acc. Chem. Res. 2003, 36, 701–712.
- 2S. Ma, Chem. Rev. 2005, 105, 2829–2872.
- 3T. Bai, S. Ma, G. Jia, Coord. Chem. Rev. 2009, 253, 423–448.
- 4S. Yu, S. Ma, Angew. Chem. Int. Ed. 2012, 51, 3074–3112 Angew. Chem. 2012, 124, 3128–3167.
- 5B. Alcaide, P. Almendros, Chem. Soc. Rev. 2014, 43, 2886.
- 6Y. Wei, M. Shi, Org. Chem. Front. 2017, 4, 1876–1890.
- 7M. Holmes, L. A. Schwartz, M. J. Krische, Chem. Rev. 2018, 118, 6026–6052.
- 8R. Blieck, M. Taillefer, F. Monnie, Chem. Rev. 2020, 120, 13545–13598.
- 9D. Campeau, D. F. L. Rayo, A. Mansour, K. Muratov, F. Gagosz, Chem. Rev. 2021, 121, 8756–8867.
- 10W. Xiao, J. Wu, Org. Chem. Front. 2022, 9, 5053–5073.
- 11J. M. Alonso, P. Almendros, Adv. Synth. Catal. 2023, 365, 1332–1384.
- 12X. Cong, L. Huang, Z. Hou, Tetrahedron 2023, 135, 133323.
- 13P. Koschker, B. Breit, Acc. Chem. Res. 2016, 49, 1524–1536.
- 14D. Tejedor, G. Méndez-Abt, L. Cotos, F. García-Tellado, Chem. Soc. Rev. 2013, 42, 458–471.
- 15T. R. Pradhan, J. K. Park, ACS Catal. 2024, 14, 7814–7845.
- 16R. Davarnejad, Alkenes–Recent Advances, New Perspectives and Applications, IntechOpen, London 2021.
10.5772/intechopen.94671 Google Scholar
- 17J. Prunet, Angew. Chem. Int. Ed. 2003, 42, 2826–2830; Angew. Chem. 2003, 115, 2932–2936.
- 18J. G. de Vries, C. J. Elsevier, The Handbook of Homogeneous Hydrogenation, Wiley-VCH, Weinheim 2008.
- 19J.-H. Xie, S.-F. Zhu, Q.-L. Zhou, Chem. Rev. 2011, 111, 1713–1760.
- 20D. Zhao, F. Glorius, Angew. Chem. Int. Ed. 2013, 52, 9616–9618; Angew. Chem. 2013, 125, 9794–9796.
- 21W. Tang, X. Zhang, Chem. Rev. 2003, 103, 3029–3070.
- 22H. Wang, J. Wen, X. Zhang, Chem. Rev. 2021, 121, 7530–7567.
- 23Z. Zhang, N. A. Butt, W. Zhang, Chem. Rev. 2016, 116, 14769–14827.
- 24C. S. G. Seo, R. H. Morris, Organometallics 2019, 38, 47–65.
- 25W. Ai, R. Zhong, X. Liu, Q. Liu, Chem. Rev. 2019, 119, 2876–2953.
- 26J. J. Verendel, O. Pàmies, M. Diéguez, P. G. Andersson, Chem. Rev. 2014, 114, 2130–2169.
- 27F. Glorius, Org. Biomol. Chem. 2005, 3, 4171–4175.
- 28P. D. Parker, X. Hou, V. M. Dong, J. Am. Chem. Soc. 2021, 143, 6724–6745.
- 29J. Wang, Stereoselective Alkene Synthesis, Springer, Berlin 2012.
10.1007/978-3-642-31824-5 Google Scholar
- 30J. Liu, Q. Liu, R. Franke, R. Jackstell, M. Beller, J. Am. Chem. Soc. 2015, 137, 8556–8563.
- 31Z. Cheng, T. Yang, C. Li, Y. Deng, F. Zhang, P. Chen, Z. Lin, S. Ma, G. Liu, J. Am. Chem. Soc. 2023, 145, 25995–26002.
- 32Y. Wang, S. G. Scrivener, X.-D. Zuo, R. Wang, P. N. Palermo, E. Murphy, A. C. Durham, Y.-M. Wang, J. Am. Chem. Soc. 2021, 143, 14998–15004.
- 33B. J. Bloomer, I. A. Joyner, M. Garcia-Borràs, D. B. Hu, M. Garçon, A. Quest, C. U. Montero, I. F. Yu, D. S. Clark, J. F. Hartwig, J. Am. Chem. Soc. 2024, 146, 1819–1824.
- 34W. Wang, S. Hong, W. He, X. Zhang, H. Qian, S. Ma, Nat. Commun. 2024, 15, 8344.
- 35S. Li, Q. Feng, L. Song, X. Zhang, Y.-D. Wu, J. Sun, J. Am. Chem. Soc. 2024, 146, 1532–1542.
- 36H. Guo, Z. Zheng, F. Yu, S. Ma, A. Holuigue, D. S. Tromp, C. J. Elsevier, Y. Yu, Angew. Chem. Int. Ed. 2006, 45, 4997–5000; Angew. Chem. 2006, 118, 5119–5122.
- 37J. Long, L. Shi, X. Li, H. Lv, X. Zhang, Angew. Chem. Int. Ed. 2018, 57, 13248–13251; Angew. Chem. 2018, 130, 13432–13435.
- 38G. Liu, X. Yang, P. Gu, M. Wang, X. Zhang, X.-Q. Dong, J. Am. Chem. Soc. 2024, 146, 7419–7430.
- 39B. Inés, D. Palomas, S. Holle, S. Steinberg, J. A. Nicasio, M. Alcarazo, Angew. Chem. Int. Ed. 2012, 51, 12367–12369; Angew. Chem. 2012, 124, 12533–12536.
- 40P. Adler, F. Gomes, A. Fadel, N. Rabasso, Eur. J. Org. Chem. 2013, 2013, 7546–7555.
- 41Z. Chen, V. M. Dong, Nat. Commun. 2017, 8, 784.
- 42R. M. Bullock, J. G. Chen, L. Gagliardi, P. J. Chirik, O. K. Farha, C. H. Hendon, C. W. Jones, J. A. Keith, J. Klosin, S. D. Minteer, R. H. Morris, A. T. Radosevich, T. B. Rauchfuss, N. A. Strotman, A. V. Vojvodic, T. R. Ward, J. Y. Yang, Y. Surendranath, Science 2020, 369, eabc3183.
- 43T. Agapie, Coord. Chem. Rev. 2011, 255, 861–880.
- 44X. Zeng, X. Cong, Org. Chem. Front. 2015, 2, 69–72.
- 45J. Li, P. Knochel, Synthesis 2019, 51, 2100–2106.
- 46X. Zeng, Synlett 2020, 31, 205–210.
- 47X. Cong, X. Zeng, Acc. Chem. Res. 2021, 54, 2014–2026.
- 48Y. Katayama, H. Mitsunuma, M. Kanai, Synthesis 2022, 54, 1684–1694.
- 49E. N. Frankel, E. Selke, C. A. Glass, J. Am. Chem. Soc. 1968, 90, 2446–2448.
- 50M. Sodeoka, Y. Ogawa, Y. Kirio, M. Shibasaki, Chem. Pharm. Bull. 1991, 39, 309–322.
- 51M. Schneider, M. J. R. Richter, E. M. Carreira, J. Am. Chem. Soc. 2020, 142, 17802–17809.
- 52K. C. K. Swamy, A. S. Reddy, K. Sandeep, A. Kalyani, Tetrahedron Lett. 2018, 59, 419–429.
- 53S. Fu, N.-Y. Chen, X. Liu, Z. Shao, S.-P. Luo, Q. Liu, J. Am. Chem. Soc. 2016, 138, 8588–8594.
- 54N. Gorgas, J. Brünig, B. Stöger, S. Vanicek, M. Tilset, L. F. Veiros, K. Kirchner, J. Am. Chem. Soc. 2019, 141, 17452–17458.
- 55J. Luo, Y. Liang, M. Montag, Y. Diskin-Posner, L. Avram, J. Am. Chem. Soc. 2022, 144, 13266–13275.
- 56Y. Wakatsuki, H. Yamazaki, M. Nakano, Y. Yamamoto, J. Chem. Soc. Chem. Commun. 1991, 703–704.
- 57A. M. LaPointe, F. C. Rix, M. Brookhart, J. Am. Chem. Soc. 1997, 119, 906–917.
- 58Z. Chen, W. Liu, O. Daugulis, M. Brookhart, J. Am. Chem. Soc. 2016, 138, 16120–16129.
- 59M. R. Elsby, S. A. Johnson, J. Am. Chem. Soc. 2017, 139, 9401–9407.
- 60H. Ohmiya, H. Yorimitsu, K. Oshima, Angew. Chem. Int. Ed. 2005, 44, 3488–3490; Angew. Chem. 2005, 117, 3554–3556.
- 61B. Han, P. Ma, X. Cong, H. Chen, X. Zeng, J. Am. Chem. Soc. 2019, 141, 9018–9026.
- 62V. C. Gibson, C. Newton, C. Redshaw, G. A. Solan, Eur. J. Inorg. Chem. 2001, 2001, 1895–1903.
10.1002/1099-0682(200107)2001:7<1895::AID-EJIC1895>3.0.CO;2-S Google Scholar
- 63K. A. Kreisel, G. P. A. Yap, K. H. Theopold, Inorg. Chem. 2008, 47, 5293–5303.
- 64K. Namba, Y. Kishi, J. Am. Chem. Soc. 2005, 127, 15382–15383.
- 65R. A. Heintz, B. S. Haggerty, H. Wan, A. L. Rheingold, K. H. Theopold, Angew. Chem. Int. Ed. 1992, 31, 1077–1079; Angew. Chem. 1992, 104, 1100–1102.
- 66C. Jones, D. Dange, A. Stasch, J. Chem. Crystallorgr. 2012, 42, 494–497.
- 67D. Zhao, L. Candish, D. Paul, F. Glorius, ACS Catal. 2016, 6, 5978–5988.
- 68M. Melaimi, M. Soleilhavoup, G. Bertrand, Angew. Chem. Int. Ed. 2010, 49, 8810–8849; Angew. Chem. 2010, 122, 8992–9032.
- 69M. Soleilhavoup, G. Bertrand, Acc. Chem. Res. 2015, 48, 256–266.
- 70M. Melaimi, R. Jazzar, M. Soleihavoup, G. Bertrand, Angew. Chem. Int. Ed. 2017, 56, 10046–10068; Angew. Chem. 2017, 129, 10180–10203.
- 71V. Lavallo, Y. Canac, C. Präsang, B. Donnadieu, G. Bertrand, Angew. Chem. Int. Ed. 2005, 44, 5705–5709; Angew. Chem. 2005, 117, 5851–5855.
- 72R. Jazzar, M. Soleilhavoup, G. Bertrand, Chem. Rev. 2020, 120, 4141–4168.
- 73L. Zhao, X. Zeng, Chem 2022, 8, 2082–2113.
- 74J. Chu, D. Munz, R. Jazzar, M. Melaimi, G. Bertrand, J. Am. Chem. Soc. 2016, 138, 7884–7887.
- 75L. Zhao, C. Hu, X. Cong, G. Deng, L. L. Liu, M. Luo, X. Zeng, J. Am. Chem. Soc. 2021, 143, 1618–1629.
- 76L. Ling, C. Hu, L. Long, X. Zhang, L. Zhao, L. L. Liu, H. Chen, M. Luo, X. Zeng, Nat. Commun. 2023, 14, 990.
- 77F. Fan, Y. Peng, X. Zhang, S. Wang, Z. Luo, M. Luo, X. Zeng, Nat. Commun. 2024, 15, 6455.
- 78W. Xue, R. Shishido, M. Oestreich, Angew. Chem. Int. Ed. 2018, 57, 12141–12145; Angew. Chem. 2018, 130, 12318–12322.
- 79T. S. Piper, D. Lemal, G. Wilkinson, Naturwissenschaften 1956, 43, 129.
- 80J. Gao, Y. Ge, C. He, Chem. Soc. Rev. 2024, 53, 4648–4673.
- 81M. Batuecas, A. Goméz-España, F. J. Fernández-Álvarez, ChemPlusChem 2024, 89, e202400162.
- 82P. Braunstein, N. M. Boag, Angew. Chem. Int. Ed. 2001, 40, 2427–2433;
10.1002/1521-3773(20010702)40:13<2427::AID-ANIE2427>3.0.CO;2-T CAS PubMed Web of Science® Google ScholarAngew. Chem. 2001, 113, 2493–2499.
- 83A. D. Sadow, T. D. Tilley, J. Am. Chem. Soc. 2005, 127, 643–656.
- 84S. Weber, M. Glavic, B. Stöger, E. Pittenauer, M. Podewitz, L. F. Veiros, K. Kirchner, J. Am. Chem. Soc. 2021, 143, 17825–17832.
- 85E. Calimano, T. D. Tilley, J. Am. Chem. Soc. 2009, 131, 11161–11173.
- 86A. D. Ibrahim, S. W. Entsminger, L. Zhu, A. R. Fout, ACS Catal. 2016, 6, 3589–3593.
- 87B. Cheng, W. Liu, Z. Lu, J. Am. Chem. Soc. 2018, 140, 5014–5017.
- 88M. A. Larsen, C. V. Wilson, J. F. Hartwig, J. Am. Chem. Soc. 2015, 137, 8633–8643.
- 89C. Karmel, J. F. Hartwig, J. Am. Chem. Soc. 2020, 142, 10494–10505.
- 90P. Lu, X. Ren, H. Xu, D. Lu, Y. Sun, Z. Lu, J. Am. Chem. Soc. 2021, 143, 12433–12438.
- 91D. C. Najera, M. N. Peñas-Defrutos, M. García-Melchor, A. R. Fout, Inorg. Chem. 2024, 63, 17706–17713.
- 92H. Yoo, P. J. Carroll, D. H. Berry, J. Am. Chem. Soc. 2006, 128, 6038–6039.
- 93K. H. Pannell, H. K. Sharma, R. N. Kapoor, F. Cervantes-Lee, J. Am. Chem. Soc. 1997, 119, 9315–9316.
- 94S. Wang, L. Long, X. Zhang, L. Ling, H. Chen, X. Zeng, Angew. Chem. Int. Ed. 2023, 62, e202312856; Angew. Chem. 2023, 135, e202312856.
- 95W. S. Mialki, R. A. Walton, Inorg. Chem. 1982, 21, 2791–2796.
- 96W. H. Monillas, G. P. A. Yap, L. A. MacAdams, K. H. Theopold, J. Am. Chem. Soc. 2007, 129, 8090–8091.
- 97I. Y. Skobelev, V. N. Panchenko, O. Y. Lyakin, K. P. Bryliakov, V. A. Zakharov, E. P. Talsi, Organometallics 2010, 29, 2943–2950.
- 98A. Grünwald, F. W. Heinemann, D. Munz, Angew. Chem. Int. Ed. 2020, 59, 21088–21095; Angew. Chem. 2020, 132, 21274–21281.
- 99Y. Li, H. Liang, Y. Liu, J. Lin, Z. Ke, ACS Catal. 2023, 13, 13008–13020.
- 100V. Lyaskovskyy, B. de Bruin, ACS Catal. 2012, 2, 270–279.
- 101P. Chirik, R. Morris, Acc. Chem. Res. 2015, 48, 2495–2495.