Rhodium-Catalyzed Asymmetric Hydrogenation and Transfer Hydrogenation of 1,3-Dipolar Nitrones
Liren Xu
Department of Chemistry, the Grubbs Institute, and Medi-X Pingshan, Southern University of Science and Technology, Shenzhen, China
Search for more papers by this authorTilong Yang
Department of Chemistry, the Grubbs Institute, and Medi-X Pingshan, Southern University of Science and Technology, Shenzhen, China
Search for more papers by this authorHao Sun
Department of Chemistry, the Grubbs Institute, and Medi-X Pingshan, Southern University of Science and Technology, Shenzhen, China
Search for more papers by this authorJingwen Zeng
Department of Chemistry, the Grubbs Institute, and Medi-X Pingshan, Southern University of Science and Technology, Shenzhen, China
Search for more papers by this authorShuo Mu
Department of Chemistry, the Grubbs Institute, and Medi-X Pingshan, Southern University of Science and Technology, Shenzhen, China
Search for more papers by this authorProf. Xumu Zhang
Department of Chemistry, the Grubbs Institute, and Medi-X Pingshan, Southern University of Science and Technology, Shenzhen, China
Search for more papers by this authorCorresponding Author
Prof. Gen-Qiang Chen
Academy for Advanced Interdisciplinary Studies, Southern University of Science and Technology, Shenzhen, China
Search for more papers by this authorLiren Xu
Department of Chemistry, the Grubbs Institute, and Medi-X Pingshan, Southern University of Science and Technology, Shenzhen, China
Search for more papers by this authorTilong Yang
Department of Chemistry, the Grubbs Institute, and Medi-X Pingshan, Southern University of Science and Technology, Shenzhen, China
Search for more papers by this authorHao Sun
Department of Chemistry, the Grubbs Institute, and Medi-X Pingshan, Southern University of Science and Technology, Shenzhen, China
Search for more papers by this authorJingwen Zeng
Department of Chemistry, the Grubbs Institute, and Medi-X Pingshan, Southern University of Science and Technology, Shenzhen, China
Search for more papers by this authorShuo Mu
Department of Chemistry, the Grubbs Institute, and Medi-X Pingshan, Southern University of Science and Technology, Shenzhen, China
Search for more papers by this authorProf. Xumu Zhang
Department of Chemistry, the Grubbs Institute, and Medi-X Pingshan, Southern University of Science and Technology, Shenzhen, China
Search for more papers by this authorCorresponding Author
Prof. Gen-Qiang Chen
Academy for Advanced Interdisciplinary Studies, Southern University of Science and Technology, Shenzhen, China
Search for more papers by this authorGraphical Abstract
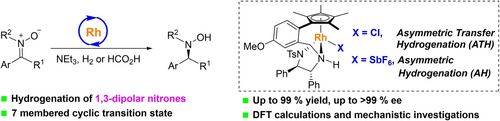
The enantioselective reduction of 1,3-dipolar nitrones to hydroxylamines was achieved by Rh(III)-catalyzed asymmetric hydrogenation and transfer hydrogenation. A wide range of chiral N,N-disubstituted hydroxylamines were synthesized with up to 99 % yield and >99 % ee. Mechanistic investigations and DFT calculations were conducted to elucidate the origin of reactivity and enantioselectivity.
Abstract
Owing to their distinctive 1,3-dipolar structure, the catalytic asymmetric hydrogenation of nitrones to hydroxylamines has been a formidable and longstanding challenge, characterized by intricate enantiocontrol and susceptibility to N−O bond cleavage. In this study, the asymmetric hydrogenation and transfer hydrogenation of nitrones were accomplished with a tethered TsDPEN-derived cyclopentadienyl rhodium(III) catalyst (TsDPEN: p-toluenesulfonyl-1,2-diphenylethylene-1,2-diamine), the reaction proceeds via a novel 7-membered cyclic transition state, producing chiral hydroxylamines with up to 99 % yield and >99 % ee. The practical viability of this methodology was underscored by gram-scale catalytic reactions and subsequent transformations. Furthermore, mechanistic investigations and DFT calculations were also conducted to elucidate the origin of enantioselectivity.
Conflict of interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202319662-sup-0001-misc_information.pdf6.4 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aL.-H. Yan, X. Li, B.-G. Wang, Nat. Prod. Rep. 2023, 10.1039/d1033np00023k;
- 1bN. Chen, J. Xie, Org. Biomol. Chem. 2016, 14, 11028–11047.
- 2M. P. Paudyal, A. M. Adebesin, S. R. Burt, D. H. Ess, Z. Ma, L. Kürti, J. R. Falck, Science 2016, 353, 1144–1147.
- 3J. Hill, T. D. Beckler, D. Crich, Molecules 2023, 28, 2816–2837.
- 4
- 4aF. Wang, Y. Chen, P. Yu, G.-Q. Chen, X. Zhang, J. Am. Chem. Soc. 2022, 144, 17763–17768;
- 4bJ. Mas-Roselló, T. Smejkal, N. Cramer, Science 2020, 368, 1098–1102;
- 4cB. Li, J. Chen, D. Liu, I. D. Gridnev, W. Zhang, Nat. Chem. 2022, 14, 920–927;
- 4dK. Yu, X. Feng, H. Du, Org. Biomol. Chem. 2022, 20, 3708–3711.
- 5
- 5aG. Wang, T. Chen, K. Jia, W. Ma, C.-H. Tung, L. Liu, J. Am. Chem. Soc. 2023, 145, 22276–22283;
- 5bG. Wang, R. Lu, C. He, L. Liu, Nat. Commun. 2021, 12, 2512–2522.
- 6S.-Q. Yang, A.-J. Han, Y. Liu, X.-Y. Tang, G.-Q. Lin, Z.-T. He, J. Am. Chem. Soc. 2023, 145, 3915–3925.
- 7
- 7aP. D. Parker, X. Hou, V. M. Dong, J. Am. Chem. Soc. 2021, 143, 6724–6745;
- 7bH. Yang, H. Yu, I. A. Stolarzewicz, W. Tang, Chem. Rev. 2023, 123, 9397–9446;
- 7cA. Cabré, X. Verdaguer, A. Riera, Chem. Rev. 2022, 122, 269–339;
- 7dR. A. A. Abdine, G. Hedouin, F. Colobert, J. Wencel-Delord, ACS Catal. 2020, 11, 215–247;
- 7eA. N. Kim, B. M. Stoltz, ACS Catal. 2020, 10, 13834–13851;
- 7fT. Ayad, P. Phansavath, V. Ratovelomanana-Vidal, Chem. Rec. 2016, 16, 2754–2771;
- 7gP.-G. Echeverria, T. Ayad, P. Phansavath, V. Ratovelomanana-Vidal, Synthesis 2016, 48, 2523–2539;
- 7hR. Molina Betancourt, P.-G. Echeverria, T. Ayad, P. Phansavath, V. Ratovelomanana-Vidal, Synthesis 2020, 53, 30–50;
- 7iD. J. Ager, A. H. M. de Vries, J. G. de Vries, Chem. Soc. Rev. 2012, 41, 3340–3380;
- 7jP. Etayo, A. Vidal-Ferran, Chem. Soc. Rev. 2013, 42, 728–754;
- 7kL. Massaro, J. Zheng, C. Margarita, P. G. Andersson, Chem. Soc. Rev. 2020, 49, 2504–2522;
- 7lJ. G. de Vries, C. J. Elsevier, Handbook of Homogeneous Hydrogenation, Wiley-VCH Verlag GmbH & Co. KGaA, 2007.
- 8
- 8aS.-I. Murahashi, Y. Imada, Chem. Rev. 2019, 119, 4684–4716;
- 8bL. Kong, X. Han, S. Liu, Y. Zou, Y. Lan, X. Li, Angew. Chem. Int. Ed. 2020, 59, 7188–7192;
- 8cF. Wang, J. Jing, Y. Zhao, X. Zhu, X.-P. Zhang, L. Zhao, P. Hu, W.-Q. Deng, X. Li, Angew. Chem. Int. Ed. 2021, 60, 16628–16633;
- 8dJ. Qi, F. Wei, S. Huang, C.-H. Tung, Z. Xu, Angew. Chem. Int. Ed. 2021, 60, 4561–4565;
- 8eJ. Qi, F. Wei, C.-H. Tung, Z. Xu, Angew. Chem. Int. Ed. 2021, 60, 13814–13818;
- 8fF. Liu, D. Qian, L. Li, X. Zhao, J. Zhang, Angew. Chem. Int. Ed. 2010, 49, 6669–6672;
- 8gF. Liu, Y. Yu, J. Zhang, Angew. Chem. Int. Ed. 2009, 48, 5505–5508;
- 8hH. Wei, M. Bao, K. Dong, L. Qiu, B. Wu, W. Hu, X. Xu, Angew. Chem. Int. Ed. 2018, 57, 17200–17204;
- 8iS. Zhou, Y. Li, X. Liu, W. Hu, Z. Ke, X. Xu, J. Am. Chem. Soc. 2021, 143, 14703–14711.
- 9
- 9aJ.-H. Xie, X.-Y. Liu, J.-B. Xie, L.-X. Wang, Q.-L. Zhou, Angew. Chem. Int. Ed. 2011, 50, 7329–7332;
- 9bC. Yin, Y.-F. Jiang, F. Huang, C.-Q. Xu, Y. Pan, S. Gao, G.-Q. Chen, X. Ding, S.-T. Bai, Q. Lang, J. Li, X. Zhang, Nat. Commun. 2023, 14, 3718–3724;
- 9cA. Fujii, S. Hashiguchi, N. Uematsu, T. Ikariya, R. Noyori, J. Am. Chem. Soc. 1996, 118, 2521–2522;
- 9dX. Li, W. Hao, N. Yi, Y.-M. He, Q.-H. Fan, CCS Chem. 2023, 5, 2277–2289.
- 10
- 10aS.-F. Zhu, J.-B. Xie, Y.-Z. Zhang, S. Li, Q.-L. Zhou, J. Am. Chem. Soc. 2006, 128, 12886–12891;
- 10bZ. Han, Z. Wang, X. Zhang, K. Ding, Angew. Chem. Int. Ed. 2009, 48, 5345–5349;
- 10cC. Li, B. Villa-Marcos, J. Xiao, J. Am. Chem. Soc. 2009, 131, 6967–6969;
- 10dZ.-Y. Ding, F. Chen, J. Qin, Y.-M. He, Q.-H. Fan, Angew. Chem. Int. Ed. 2012, 51, 5706–5710;
- 10eC. Guo, D.-W. Sun, S. Yang, S.-J. Mao, X.-H. Xu, S.-F. Zhu, Q.-L. Zhou, J. Am. Chem. Soc. 2015, 137, 90–93;
- 10fJ. Chen, Z. Zhang, B. Li, F. Li, Y. Wang, M. Zhao, I. D. Gridnev, T. Imamoto, W. Zhang, Nat. Commun. 2018, 9, 5000–5009;
- 10gC. Liu, M. Wang, Y. Xu, Y. Li, Q. Liu, Angew. Chem. Int. Ed. 2022, 61, e202202814;
- 10hY. Gao, Z. Wang, X. Zhang, M. Zhao, S. Zhang, C. Wang, L. Xu, P. Li, Angew. Chem. Int. Ed. 2023, 62, e2023037;
- 10iZ. Yang, F. Chen, Y. He, N. Yang, Q.-H. Fan, Angew. Chem. Int. Ed. 2016, 55, 13863–13866;
- 10jT. Touge, T. Arai, J. Am. Chem. Soc. 2016, 138, 11299–11305;
- 10kR. A. A. Abdine, G. Hedouin, F. Colobert, J. Wencel-Delord, ACS Catal. 2021, 11, 215–247;
- 10lN. Fleury-Brégeot, V. de la Fuente, S. Castillón, C. Claver, ChemCatChem 2010, 2, 1346–1371.
- 11
- 11aS.-I. Murahashi, T. Tsuji, S. Ito, Chem. Commun. 2000, 409–410;
- 11bS.-I. Murahashi, S. Watanabe, T. Shiota, J. Chem. Soc. Chem. Commun. 1994, 725–726.
- 12P.-W. Xu, X.-Y. Cui, C. Chen, F. Zhou, J.-S. Yu, Y.-F. Ao, J. Zhou, Org. Lett. 2021, 23, 8471–8476.
- 13A. Y. Sukhorukov, Adv. Synth. Catal. 2019, 362, 724–754.
- 14
- 14aK. Huang, S. Li, M. Chang, X. Zhang, Org. Lett. 2013, 15, 484–487;
- 14bP. Krasik, H. Alper, Tetrahedron: Asymmetry 1992, 3, 1283–1288;
- 14cY. Xie, A. Mi, Y. Jiang, H. Liu, Synth. Commun. 2001, 31, 2767–2771;
- 14dY. Chu, Z. Shan, D. Liu, N. Sun, J. Org. Chem. 2006, 71, 3998–4001.
- 15
- 15aJ. Trouvé, R. Gramage-Doria, Chem. Soc. Rev. 2021, 50, 3565–3584;
- 15bJ. N. H. Reek, B. de Bruin, S. Pullen, T. J. Mooibroek, A. M. Kluwer, X. Caumes, Chem. Rev. 2022, 122, 12308–12369;
- 15cW. Tang, S. Johnston, J. A. Iggo, N. G. Berry, M. Phelan, L. Lian, J. Bacsa, J. Xiao, Angew. Chem. Int. Ed. 2013, 52, 1668–1672;
- 15dP. A. Dub, J. C. Gordon, ACS Catal. 2017, 7, 6635–6655;
- 15eL.-S. Zheng, Q. Llopis, P.-G. Echeverria, C. Férard, G. Guillamot, P. Phansavath, V. Ratovelomanana-Vidal, J. Org. Chem. 2017, 82, 5607–5615;
- 15fF. Wang, T. Yang, T. Wu, L.-S. Zheng, C. Yin, Y. Shi, X.-Y. Ye, G.-Q. Chen, X. Zhang, J. Am. Chem. Soc. 2021, 143, 2477–2483;
- 15gF. Wang, Z. Zhang, Y. Chen, V. Ratovelomanana-Vidal, P. Yu, G.-Q. Chen, X. Zhang, Nat. Commun. 2022, 13, 7794–7803;
- 15hT. Chen, W. Liu, W. Gu, S. Niu, S. Lan, Z. Zhao, F. Gong, J. Liu, S. Yang, A. E. Cotman, J. Song, X. Fang, J. Am. Chem. Soc. 2023, 145, 585–599.
- 16
- 16aH. Wang, J. Wen, X. Zhang, Chem. Rev. 2021, 121, 7530–7567;
- 16bW. Wu, S. Liu, M. Duan, X. Tan, C. Chen, Y. Xie, Y. Lan, X.-Q. Dong, X. Zhang, Org. Lett. 2016, 18, 2938–2941;
- 16cX. Zhang, P.-L. Shao, in Asymmetric Hydrogenation and Transfer Hydrogenation, 2021, pp. 175–219.
10.1002/9783527822294.ch6 Google Scholar
- 17
- 17aF. Wang, L.-S. Zheng, Q.-W. Lang, C. Yin, T. Wu, P. Phansavath, G.-Q. Chen, V. Ratovelomanana-Vidal, X. Zhang, Chem. Commun. 2020, 56, 3119–3122;
- 17bV. Ritleng, J. G. de Vries, Molecules 2021, 26, 4076–4120.
- 18
- 18aX. Wu, J. Liu, D. Di Tommaso, J. A. Iggo, C. R. Catlow, J. Bacsa, J. Xiao, Chem. Eur. J. 2008, 14, 7699–7715;
- 18bJ. Hannedouche, G. J. Clarkson, M. Wills, J. Am. Chem. Soc. 2004, 126, 986–987;
- 18cA. M. Hayes, D. J. Morris, G. J. Clarkson, M. Wills, J. Am. Chem. Soc. 2005, 127, 7318–7319.
- 19
- 19aA. M. R. Hall, D. B. G. Berry, J. N. Crossley, A. Codina, I. Clegg, J. P. Lowe, A. Buchard, U. Hintermair, ACS Catal. 2021, 11, 13649–13659;
- 19bP.-G. Echeverria, C. Férard, P. Phansavath, V. Ratovelomanana-Vidal, Catal. Commun. 2015, 62, 95–99.
- 20C. Li, J. Xiao, J. Am. Chem. Soc. 2008, 130, 13208–13209.
- 21Deposition Number 2290753 contains the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service.
- 22F. Cheng, D. Li, J. Li, Y. Tang, Y. Wu, S. Xu, Org. Lett. 2023, 25, 2555–2559.
- 23
- 23aR. Y. Liu, S. L. Buchwald, Acc. Chem. Res. 2020, 53, 1229–1243;
- 23bK. Hirano, M. Miura, J. Am. Chem. Soc. 2022, 144, 648–661.
- 24Y.-B. Li, H. Tian, S. Zhang, J.-Z. Xiao, L. Yin, Angew. Chem. Int. Ed. 2022, 61, e202117760.
- 25Y. Yuan, Y. Zhang, W. Li, Y. Zhao, X.-F. Wu, Angew. Chem. Int. Ed. 2023, 62, e202309993.
- 26A. J. Martín, S. Mitchell, C. Mondelli, S. Jaydev, J. Pérez-Ramírez, Nat. Catal. 2022, 5, 854–866.
- 27H. F. Motiwala, A. M. Armaly, J. G. Cacioppo, T. C. Coombs, K. R. K. Koehn, V. M. t. Norwood, J. Aubé, Chem. Rev. 2022, 122, 12544–12747.