Flavin-N5OOH Functions as both a Powerful Nucleophile and a Base in the Superfamily of Flavoenzymes
Qiaoyu Zhang
State Key Laboratory of Physical Chemistry of Solid Surfaces and Fujian Provincial Key Laboratory of Theoretical and Computational Chemistry, College of Chemistry and Chemical Engineering, Xiamen University, Xiamen, 361005 P. R. China
Search for more papers by this authorQianqian Chen
State Key Laboratory of Physical Chemistry of Solid Surfaces and Fujian Provincial Key Laboratory of Theoretical and Computational Chemistry, College of Chemistry and Chemical Engineering, Xiamen University, Xiamen, 361005 P. R. China
Search for more papers by this authorCorresponding Author
Prof. Sason Shaik
Institute of Chemistry and the Lise Meitner-Minerva Center for Computational Quantum Chemistry, The Hebrew University of Jerusalem, 91904 Jerusalem, Israel
Search for more papers by this authorCorresponding Author
Prof. Binju Wang
State Key Laboratory of Physical Chemistry of Solid Surfaces and Fujian Provincial Key Laboratory of Theoretical and Computational Chemistry, College of Chemistry and Chemical Engineering, Xiamen University, Xiamen, 361005 P. R. China
Search for more papers by this authorQiaoyu Zhang
State Key Laboratory of Physical Chemistry of Solid Surfaces and Fujian Provincial Key Laboratory of Theoretical and Computational Chemistry, College of Chemistry and Chemical Engineering, Xiamen University, Xiamen, 361005 P. R. China
Search for more papers by this authorQianqian Chen
State Key Laboratory of Physical Chemistry of Solid Surfaces and Fujian Provincial Key Laboratory of Theoretical and Computational Chemistry, College of Chemistry and Chemical Engineering, Xiamen University, Xiamen, 361005 P. R. China
Search for more papers by this authorCorresponding Author
Prof. Sason Shaik
Institute of Chemistry and the Lise Meitner-Minerva Center for Computational Quantum Chemistry, The Hebrew University of Jerusalem, 91904 Jerusalem, Israel
Search for more papers by this authorCorresponding Author
Prof. Binju Wang
State Key Laboratory of Physical Chemistry of Solid Surfaces and Fujian Provincial Key Laboratory of Theoretical and Computational Chemistry, College of Chemistry and Chemical Engineering, Xiamen University, Xiamen, 361005 P. R. China
Search for more papers by this authorGraphical Abstract
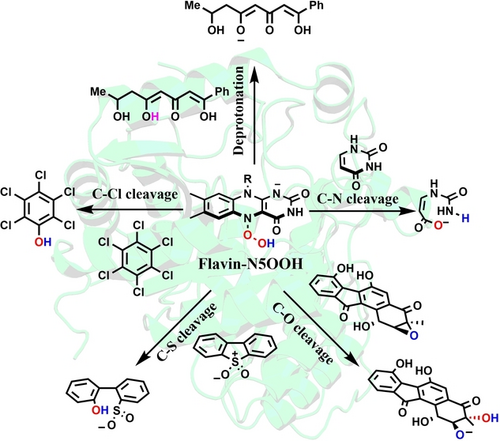
A computational study demonstrates that Flavin-N5OOH species can function as both powerful nucleophiles and bases in the superfamily of flavoenzymes, which can mediate many kinds of challenging non-redox reactions, such as the cleavage of C−X (X=N, O, S, Cl) bonds. The findings revive oxygen activation chemistry by flavoenzymes and are expected to spur the discovery of many other Flavin-N5OOH-dependent enzymes.
Abstract
Flavoenzymes can mediate a large variety of oxidation reactions through the activation of oxygen. However, the O2 activation chemistry of flavin enzymes is not yet fully exploited. Normally, the O2 activation occurs at the C4a site of the flavin cofactor, yielding the flavin C4a-(hydro)hydroperoxyl species in monooxygenases or oxidases. Using extensive MD simulations, QM/MM calculations and QM calculations, our studies reveal the formation of the common nucleophilic species, Flavin-N5OOH, in two distinct flavoenzymes (RutA and EncM). Our studies show that Flavin-N5OOH acts as a powerful nucleophile that promotes C−N cleavage of uracil in RutA, and a powerful base in the deprotonation of substrates in EncM. We reason that Flavin-N5OOH can be a common reactive species in the superfamily of flavoenzymes, which accomplish generally selective general base catalysis and C−X (X=N, S, Cl, O) cleavage reactions that are otherwise challenging with solvated hydroxide ion base. These results expand our understanding of the chemistry and catalysis of flavoenzymes.
Conflict of interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202318629-sup-0001-misc_information.pdf2.8 MB | Supporting Information |
| anie202318629-sup-0001-Movie-S1.mp43.6 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aP. Macheroux, B. Kappes, S. E. Ealick, FEBS J. 2011, 278, 2625–2634;
- 1bC. T. Walsh, T. A. Wencewicz, Nat. Prod. Rep. 2013, 30, 175–200;
- 1cT. Heine, W. J. H. van Berkel, G. Gassner, K. H. van Pee, D. Tischler, Biology 2018, 7, 42;
- 1dR. Teufel, Arch. Biochem. Biophys. 2017, 632, 20–27;
- 1eR. Teufel, V. Agarwal, B. S. Moore, Curr. Opin. Chem. Biol. 2016, 31, 31–39;
- 1fV. Massey, J. Biol. Chem. 1994, 269, 22459–22462;
- 1gE. Romero, J. R. Gómez Castellanos, G. Gadda, M. W. Fraaije, A. Mattevi, Chem. Rev. 2018, 118, 1742–1769;
- 1hS. Ghisla, V. Massey, Eur. J. Biochem. 1989, 181, 1–17;
- 1iT. C. Bruice, Acc. Chem. Res. 1980, 13, 256–262;
- 1jS. O. Mansoorabadi, C. J. Thibodeaux, H.-w. Liu, J. Org. Chem. 2007, 72, 6329–6342.
- 2
- 2aM. M. Huijbers, S. Montersino, A. H. Westphal, D. Tischler, W. J. van Berkel, Arch. Biochem. Biophys. 2014, 544, 2–17;
- 2bP. Chaiyen, M. W. Fraaije, A. Mattevi, Trends Biochem. Sci. 2012, 37, 373–380.
- 3
- 3aP. F. Fitzpatrick, Acc. Chem. Res. 2001, 34, 299–307;
- 3bS. Ghisla, C. Thorpe, Eur. J. Biochem. 2004, 271, 494–508;
- 3cD. Ouedraogo, J. Ball, A. Iyer, R. A. G. Reis, M. Vodovoz, G. Gadda, Arch. Biochem. Biophys. 2017, 632, 192–201.
- 4
- 4aC. E. Paul, D. Eggerichs, A. H. Westphal, D. Tischler, W. J. H. van Berkel, Biotechnol. Adv. 2021, 51, 107712;
- 4bW. J. van Berkel, N. M. Kamerbeek, M. W. Fraaije, J. Biotechnol. 2006, 124, 670–689;
- 4cH. Leisch, K. Morley, P. C. Lau, Chem. Rev. 2011, 111, 4165–4222;
- 4dG. de Gonzalo, M. D. Mihovilovic, M. W. Fraaije, ChemBioChem 2010, 11, 2208–2231;
- 4eH. R. Ellis, Arch. Biochem. Biophys. 2010, 497, 1–12;
- 4fP. Chenprakhon, T. Wongnate, P. Chaiyen, Protein Sci. 2019, 28, 8–29.
- 5
- 5aM. C. Andorfer, J. C. Lewis, Annu. Rev. Biochem. 2018, 87, 159–185;
- 5bA. Phintha, K. Prakinee, P. Chaiyen, Enzymes 2020, 47, 327–364;
- 5cV. Agarwal, Z. D. Miles, J. M. Winter, A. S. Eustaquio, A. A. El Gamal, B. S. Moore, Chem. Rev. 2017, 117, 5619–5674;
- 5dL. C. Blasiak, C. L. Drennan, Acc. Chem. Res. 2009, 42, 147–155;
- 5eK. Prakinee, A. Phintha, S. Visitsatthawong, N. Lawan, J. Sucharitakul, C. Kantiwiriyawanitch, J. Damborsky, P. Chitnumsub, K.-H. van Pée, P. Chaiyen, Nat. Catal. 2022, 5, 534–544;
- 5fR. D. Barker, Y. Yu, L. De Maria, L. O. Johannissen, N. S. Scrutton, ACS Catal. 2022, 12, 15352–15360.
- 6
- 6aD. Sorigué, B. Légeret, S. Cuiné, S. Blangy, S. Moulin, E. Billon, P. Richaud, S. Brugière, Y. Couté, D. Nurizzo, P. Müller, K. Brettel, D. Pignol, P. Arnoux, Y. Li-Beisson, G. Peltier, F. Beisson, Science 2017, 357, 903–907;
- 6bP. F. Heelis, Chem. Soc. Rev. 1982, 11, 15–39;
- 6cW. Harrison, X. Huang, H. Zhao, Acc. Chem. Res. 2022, 55, 1087–1096;
- 6dB. A. Sandoval, S. I. Kurtoic, M. M. Chung, K. F. Biegasiewicz, T. K. Hyster, Angew. Chem. Int. Ed. Engl. 2019, 58, 8714–8718;
- 6eX. Huang, B. Wang, Y. Wang, G. Jiang, J. Feng, H. Zhao, Nature 2020, 584, 69–74.
- 7
- 7aE. I. Solomon, D. E. Heppner, E. M. Johnston, J. W. Ginsbach, J. Cirera, M. Qayyum, M. T. Kieber-Emmons, C. H. Kjaergaard, R. G. Hadt, L. Tian, Chem. Rev. 2014, 114, 3659–3853;
- 7bX. Huang, J. T. Groves, Chem. Rev. 2018, 118, 2491–2553;
- 7cS. Shaik, S. Cohen, Y. Wang, H. Chen, D. Kumar, W. Thiel, Chem. Rev. 2010, 110, 949–1017.
- 8
- 8aC. Walsh, Acc. Chem. Res. 1980, 13, 148–155;
- 8bQ. Wang, N. Liu, Y. Deng, Y. Guan, H. Xiao, T. A. Nitka, H. Yang, A. Yadav, L. Vukovic, Mathews II, X. Chen, C. Y. Kim, Nat. Commun. 2023, 14, 6273;
- 8cA. M. Chanique, N. Polidori, L. Sovic, D. Kracher, L. Assil-Companioni, P. Galuska, L. P. Parra, K. Gruber, R. Kourist, ACS Catal. 2023, 13, 3549–3562;
- 8dC. H. Chiang, T. Wymore, A. Rodriguez Benitez, A. Hussain, J. L. Smith, C. L. Brooks III, A. R. H. Narayan, Proc. Natl. Acad. Sci. USA 2023, 120, e2218248120;
- 8eD. K. Liscombe, Y. Kamiyoshihara, J. Ghironzi, C. J. Kempthorne, K. Hooton, B. Bulot, V. Kanellis, J. McNulty, N. B. Lam, L. F. Nadeau, M. Pautler, D. M. Tieman, H. J. Klee, C. Goulet, Proc. Natl. Acad. Sci. USA 2022, 119, e2118676119;
- 8fS. Hazra, T. P. Begley, J. Am. Chem. Soc. 2023, 145, 11933–11938;
- 8gD. Zabala, J. W. Cartwright, D. M. Roberts, B. J. Law, L. Song, M. Samborskyy, P. F. Leadlay, J. Micklefield, G. L. Challis, J. Am. Chem. Soc. 2016, 138, 4342–4345;
- 8hP. Pimviriyakul, P. Surawatanawong, P. Chaiyen, Chem. Sci. 2018, 9, 7468–7482;
- 8iR. A. G. Reis, H. Li, M. Johnson, P. Sobrado, Arch. Biochem. Biophys. 2021, 699, 108765;
- 8jP. Pimviriyakul, P. Chaiyen, Enzymes 2020, 47, 1–36;
- 8kY. Hu, D. Dietrich, W. Xu, A. Patel, J. A. Thuss, J. Wang, W. B. Yin, K. Qiao, K. N. Houk, J. C. Vederas, Y. Tang, Nat. Chem. Biol. 2014, 10, 552–554;
- 8lM. J. H. Moonen, M. W. Fraaije, I. M. C. M. Rietjens, C. Laane, W. J. H. van Berkel, Adv. Synth. Catal. 2002, 344, 1023–1035;
- 8mM. D. Mihovilovic, B. Müller, P. Stanetty, Eur. J. Org. Chem. 2002, 2002, 3711–3730;
- 8nK. A. Hicks, M. E. Yuen, W. F. Zhen, T. J. Gerwig, R. W. Story, M. C. Kopp, M. J. Snider, Biochemistry 2016, 55, 3432–3446;
- 8oA. R. Benitez, S. Tweedy, S. A. Baker Dockrey, A. L. Lukowski, T. Wymore, D. Khare, C. L. Brooks III, B. A. Palfey, J. L. Smith, A. R. H. Narayan, ACS Catal. 2019, 9, 3633–3640.
- 9
- 9aY. Luo, Y.-J. Liu, ChemPhysChem 2019, 20, 405–409;
- 9bS. Badieyan, R. D. Bach, P. Sobrado, J. Org. Chem. 2015, 80, 2139–2147;
- 9cB. A. Palfey, C. A. McDonald, Arch. Biochem. Biophys. 2010, 493, 26–36;
- 9dA. Mattevi, Trends Biochem. Sci. 2006, 31, 276–283;
- 9eM. W. Fraaije, A. Mattevi, Trends Biochem. Sci. 2000, 25, 126–132;
- 9fI. Polyak, M. T. Reetz, W. Thiel, J. Am. Chem. Soc. 2012, 134, 2732–2741;
- 9gS. E. Tweedy, A. Rodriguez Benitez, A. R. H. Narayan, P. M. Zimmerman, C. L. Brooks III, T. Wymore, J. Phys. Chem. B 2019, 123, 8065–8073;
- 9hA. C. C. Barbosa, R. P. P. Neves, S. F. Sousa, M. J. Ramos, P. A. Fernandes, ACS Catal. 2018, 8, 9298–9311;
- 9iM. J. L. J. Fürst, A. Gran-Scheuch, F. S. Aalbers, M. W. Fraaije, ACS Catal. 2019, 9, 11207–11241;
- 9jL. Ridder, A. J. Mulholland, I. M. C. M. Rietjens, J. Vervoort, J. Am. Chem. Soc. 2000, 122, 8728–8738;
- 9kL. Ridder, J. N. Harvey, I. M. C. M. Rietjens, J. Vervoort, A. J. Mulholland, J. Phys. Chem. B 2003, 107, 2118–2126.
- 10
- 10aR. Teufel, Meth. Enzymol. 2018, 604, 523–540;
- 10bR. Saleem-Batcha, F. Stull, J. N. Sanders, B. S. Moore, B. A. Palfey, K. N. Houk, R. Teufel, Proc. Natl. Acad. Sci. USA 2018, 115, 4909–4914;
- 10cR. Teufel, F. Stull, M. J. Meehan, Q. Michaudel, P. C. Dorrestein, B. Palfey, B. S. Moore, J. Am. Chem. Soc. 2015, 137, 8078–8085;
- 10dR. Teufel, A. Miyanaga, Q. Michaudel, F. Stull, G. Louie, J. P. Noel, P. S. Baran, B. Palfey, B. S. Moore, Nature 2013, 503, 552–556;
- 10eQ. Cheng, L. Xiang, M. Izumikawa, D. Meluzzi, B. S. Moore, Nat. Chem. Biol. 2007, 3, 557–558;
- 10fL. Xiang, J. A. Kalaitzis, B. S. Moore, Proc. Natl. Acad. Sci. USA 2004, 101, 15609–15614.
- 11
- 11aA. Matthews, R. Saleem-Batcha, J. N. Sanders, F. Stull, K. N. Houk, R. Teufel, Nat. Chem. Biol. 2020, 16, 556–563;
- 11bS. Adak, T. P. Begley, Biochemistry 2017, 56, 3708–3709.
- 12
- 12aY. Duan, M. Toplak, A. Hou, N. L. Brock, J. S. Dickschat, R. Teufel, J. Am. Chem. Soc. 2021, 143, 10413–10421;
- 12bM. Toplak, A. Matthews, R. Teufel, Arch. Biochem. Biophys. 2021, 698, 108732;
- 12cA. Matthews, J. Schonfelder, S. Lagies, E. Schleicher, B. Kammerer, H. R. Ellis, F. Stull, R. Teufel, FEBS J. 2022, 289, 787–807;
- 12dM. Toplak, R. Teufel, Biochemistry 2022, 61, 47–56.
- 13C. Walsh, J. Fisher, R. Spencer, D. W. Graham, W. T. Ashton, J. E. Brown, R. D. Brown, E. F. Rogers, Biochemistry 1978, 17, 1942–1951.
- 14B. Wang, Z. Cao, D. A. Sharon, S. Shaik, ACS Catal. 2015, 5, 7077–7090.
- 15
- 15aJ. R. Pliego, J. M. Riveros, J. Phys. Chem. A 2002, 106, 7434–7439;
- 15bB. Wang, Z. Cao, J. Phys. Chem. A 2010, 114, 12918–12927;
- 15cB. Wang, Z. Cao, Angew. Chem. Int. Ed. 2011, 50, 3266–3270;
- 15dB. Wang, Z. Cao, J. Comput. Chem. 2013, 34, 372–378;
- 15eJ. R. Pliego, J. M. Riveros, WIREs Comput. Mol. Sci. 2020, 10, e1440.
- 16M. Tuckerman, K. Laasonen, M. Sprik, M. Parrinello, J. Phys. Chem. C 1995, 99, 5749–5752.
- 17S. Adak, T. P. Begley, J. Am. Chem. Soc. 2016, 138, 6424–6426.
- 18
- 18aS. Adak, T. P. Begley, Biochemistry 2019, 58, 1181–1183;
- 18bK. Takagi, A. Iwasaki, I. Kamei, K. Satsuma, Y. Yoshioka, N. Harada, Appl. Environ. Microbiol. 2009, 75, 4452–4458.
- 19C. Yang, L. Zhang, W. Zhang, C. Huang, Y. Zhu, X. Jiang, W. Liu, M. Zhao, B. C. De, C. Zhang, Nat. Commun. 2022, 13, 5386.
- 20
- 20aT. Wongnate, P. Surawatanawong, S. Visitsatthawong, J. Sucharitakul, N. S. Scrutton, P. Chaiyen, J. Am. Chem. Soc. 2013, 136, 241–253;
- 20bB. Martin Hallberg, C. Leitner, D. Haltrich, C. Divne, J. Mol. Biol. 2004, 341, 781–796.
- 21
- 21aA. Alfieri, F. Fersini, N. Ruangchan, M. Prongjit, P. Chaiyen, A. Mattevi, Proc. Natl. Acad. Sci. USA 2007, 104, 1177–1182;
- 21bP. Chenprakhon, D. Trisrivirat, K. Thotsaporn, J. Sucharitakul, P. Chaiyen, Biochemistry 2014, 53, 4084–4086;
- 21cS. Visitsatthawong, P. Chenprakhon, P. Chaiyen, P. Surawatanawong, J. Am. Chem. Soc. 2015, 137, 9363–9374.
- 22
- 22aL. J. Guan, W. C. Lee, S. Wang, T. Ohshiro, Y. Izumi, J. Ohtsuka, M. Tanokura, FEBS J. 2015, 282, 3126–3135;
- 22bS. Liu, C. Zhang, T. Su, T. Wei, D. Zhu, K. Wang, Y. Huang, Y. Dong, K. Yin, S. Xu, P. Xu, L. Gu, Proteins Struct. Funct. Bioinf. 2014, 82, 1708–1720.