SN2 Reaction at the Amide Nitrogen Center Enables Hydrazide Synthesis
Wen Fang
School of Food and Biological Engineering, Hefei University of Technology, Hefei, China
These authors contributed equally to this work
Search for more papers by this authorZhi-Wen Luo
School of Food and Biological Engineering, Hefei University of Technology, Hefei, China
These authors contributed equally to this work
Search for more papers by this authorDr. Ye-Cheng Wang
Department of Chemistry and Center for Cancer Research, Purdue University, West Lafayette, IN, USA
These authors contributed equally to this work
Search for more papers by this authorWei Zhou
School of Food and Biological Engineering, Hefei University of Technology, Hefei, China
Search for more papers by this authorDr. Lei Li
Department of Chemistry, Emory University, Atlanta, GA, USA
Search for more papers by this authorYimin Chen
Department of Chemistry, University of Pittsburgh, Pittsburgh, PA, USA
Search for more papers by this authorXiangke Zhang
Department of Chemistry and Center for Cancer Research, Purdue University, West Lafayette, IN, USA
Search for more papers by this authorCorresponding Author
Prof. Dr. Mingji Dai
Department of Chemistry, Emory University, Atlanta, GA, USA
Department of Chemistry and Center for Cancer Research, Purdue University, West Lafayette, IN, USA
Search for more papers by this authorCorresponding Author
Prof. Dr. Jian-Jun Dai
School of Food and Biological Engineering, Hefei University of Technology, Hefei, China
Search for more papers by this authorWen Fang
School of Food and Biological Engineering, Hefei University of Technology, Hefei, China
These authors contributed equally to this work
Search for more papers by this authorZhi-Wen Luo
School of Food and Biological Engineering, Hefei University of Technology, Hefei, China
These authors contributed equally to this work
Search for more papers by this authorDr. Ye-Cheng Wang
Department of Chemistry and Center for Cancer Research, Purdue University, West Lafayette, IN, USA
These authors contributed equally to this work
Search for more papers by this authorWei Zhou
School of Food and Biological Engineering, Hefei University of Technology, Hefei, China
Search for more papers by this authorDr. Lei Li
Department of Chemistry, Emory University, Atlanta, GA, USA
Search for more papers by this authorYimin Chen
Department of Chemistry, University of Pittsburgh, Pittsburgh, PA, USA
Search for more papers by this authorXiangke Zhang
Department of Chemistry and Center for Cancer Research, Purdue University, West Lafayette, IN, USA
Search for more papers by this authorCorresponding Author
Prof. Dr. Mingji Dai
Department of Chemistry, Emory University, Atlanta, GA, USA
Department of Chemistry and Center for Cancer Research, Purdue University, West Lafayette, IN, USA
Search for more papers by this authorCorresponding Author
Prof. Dr. Jian-Jun Dai
School of Food and Biological Engineering, Hefei University of Technology, Hefei, China
Search for more papers by this authorGraphical Abstract
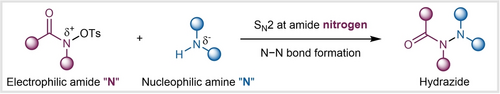
A new N−N coupling approach to the practical synthesis of hydrazides is disclosed by employing an SN2 strategy at electrophilic amides with nucleophilic amines. The reaction exhibits mild conditions, broad substrate scope, excellent functional group tolerance, easy scalability, and is applicable to the late-stage modification of various approved drug molecules, thus enabling complex hydrazide scaffold synthesis.
Abstract
Nucleophilic substitutions are fundamentally important transformations in synthetic organic chemistry. Despite the substantial advances in bimolecular nucleophilic substitutions (SN2) at saturated carbon centers, analogous SN2 reaction at the amide nitrogen atom remains extremely limited. Here we report an SN2 substitution method at the amide nitrogen atom with amine nucleophiles for nitrogen–nitrogen (N−N) bond formation that leads to a novel strategy toward biologically and medicinally important hydrazide derivatives. We found the use of sulfonate-leaving groups at the amide nitrogen atom played a pivotal role in the reaction. This new N−N coupling reaction allows the use of O-tosyl hydroxamates as electrophiles and readily available amines, including acyclic aliphatic amines and saturated N-heterocycles as nucleophiles. The reaction features mild conditions, broad substrate scope (>80 examples), excellent functional group tolerability, and scalability. The method is applicable to late-stage modification of various approved drug molecules, thus enabling complex hydrazide scaffold synthesis.
Conflict of interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202317570-sup-0001-misc_information.pdf22.1 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aL. M. Blair, J. Sperry, J. Nat. Prod. 2013, 76, 794–812;
- 1bA. J. Waldman, T. L. Ng, P. Wang, E. P. Balskus, Chem. Rev. 2017, 117, 5784–5863;
- 1cB. R. Rosen, E. W. Werner, A. G. O'Brien, P. S. Baran, J. Am. Chem. Soc. 2014, 136, 5571–5574;
- 1dA. Tabey, P. Y. Vemuri, F. W. Patureau, Chem. Sci. 2021, 12, 14343–14352;
- 1eJ. C. Bieniek, M. Grünewald, J. Winter, D. Schollmeyer, S. R. Waldvogel, Chem. Sci. 2022, 13, 8180–8186.
- 2
- 2aY.-L. Du, H.-Y. He, M. A. Higgins, K. S. Ryan, Nat. Chem. Biol. 2017, 13, 836–838;
- 2bS.-Y. Yin, Q. Zhou, C.-X. Liu, Q. Gu, S.-L. You, Angew. Chem. Int. Ed. 2023, 62, e202305067;
- 2cL. Chen, Z. Deng, C. Zhao, ACS Chem. Biol. 2021, 16, 559–570.
- 3
- 3aY. Kawase, T. Yamagishi, J. Kato, T. Kutsuma, T. Kataoka, T. Iwakuma, T. Yokomatsu, Synthesis 2014, 46, 455–464;
- 3bZ.-Q. Long, L.-L. Yang, J.-R. Zhang, S.-T. Liu, J. Xie, P.-Y. Wang, J.-J. Zhu, W.-B. Shao, L.-W. Liu, S. Yang, J. Agric. Food Chem. 2021, 69, 8380–8393;
- 3cP. Majumdar, A. Pati, M. Patra, R. K. Behera, A. K. Behera, Chem. Rev. 2014, 114, 2942–2977.
- 4J.-P. Schirmann, P. Bourdauducq, “Hydrazine”. Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH, 2001.
- 5
- 5aJ. Vidal, J.-C. Hannachi, G. Hourdin, J.-C. Mulatier, A. Collet, Tetrahedron Lett. 1998, 39, 8845–8848;
- 5bH. Wang, H. Jung, F. Song, S. Zhu, Z. Bai, D. Chen, G. He, S. Chang, G. Chen, Nat. Chem. 2021, 13, 378–385;
- 5cJ. P. Barbor, V. N. Nair, K. R. Sharp, T. D. Lohrey, S. E. Dibrell, T. K. Shah, M. J. Walsh, S. E. Reisman, B. M. Stoltz, J. Am. Chem. Soc. 2023, 145, 15071–15077.
- 6
- 6aW. A. Cowdrey, E. D. Hughes, C. K. Ingold, Nature 1936, 138, 759;
- 6bE. D. Hughes, C. K. Ingold, A. D. Scott, J. Chem. Soc. 1937, 1201–1208;
- 6cE. D. Hughes, C. K. Ingold, R. J. L. Martin, D. F. Meigh, Nature 1950, 166, 679–680;
- 6dX. Zhang, C.-H. Tan, Chem 2021, 7, 1451–1486;
- 6eT. A. Hamlin, M. Swart, F. M. Bickelhaupt, ChemPhysChem 2018, 19, 1315–1330.
- 7
- 7aG. X. Ortiz Jr, B. N. Hemric, Q. Wang, Org. Lett. 2017, 19, 1314–1317;
- 7bC. Greck, L. Bischoff, A. Girard, J. Hajicek, J. P. Genet, Bull. Soc. Chim. Fr. 1994, 131, 429–433;
- 7cX. Ma, J. J. Farndon, T. A. Young, N. Fey, J. F. Bower, Angew. Chem. Int. Ed. 2017, 56, 14531–14535;
- 7dJ. J. Farndon, X. Ma, J. F. Bower, J. Am. Chem. Soc. 2017, 139, 14005–14008;
- 7eX. Dong, Q. Liu, Y. Dong, H. Liu, Chem. Eur. J. 2017, 23, 2481–2511;
- 7fM. Corpet, C. Gosmini, Synthesis 2014, 46, 2258–2271;
- 7gI. R. Hazelden, R. C. Carmona, T. Langer, P. G. Pringle, J. F. Bower, Angew. Chem. Int. Ed. 2018, 57, 5124–5128;
- 7hX. Ma, I. R. Hazelden, T. Langer, R. H. Munday, J. F. Bower, J. Am. Chem. Soc. 2019, 141, 3356–3360;
- 7iB. T. Jones, J. García-Cárceles, L. Caiger, I. R. Hazelden, R. J. Lewis, T. Langer, J. F. Bower, J. Am. Chem. Soc. 2021, 143, 15593–15598;
- 7jL. G. O′Neil, J. F. Bower, Angew. Chem. Int. Ed. 2021, 60, 25640–25666;
- 7kY.-K. Mei, X.-T. Min, S.-Y. Guo, C.-H. Liu, X.-X. Zhang, D.-W. Ji, B Wan, Q.-A. Chen, Eur. J. Org. Chem. 2022, 2022, e202200043;
- 7lY. Zhu, M. J. S. Smith, W. Tu, J. F. Bower, Angew. Chem. Int. Ed. 2023, 62, e202301262.
- 8
- 8aS. A. Glover, Tetrahedron 1998, 54, 7229–7271;
- 8bS. A. Glover, Arkivoc 2001, 12, 143–160;
- 8cS. A. Glover, G. Mo, J. Chem. Soc. Perkin Trans. 2 2002, 1728–1739;
- 8dK. L. Cavanagh, S. A. Glover, H. L. Price, R. R. Schumacher, Aust. J. Chem. 2009, 62, 700–710;
- 8eS. A. Glover, A. A. Rosser, Molecules 2018, 23, 2834.
- 9S. H. Kennedy, B. D. Dherange, K. J. Berger, M. D. Levin, Nature 2021, 593, 223–227.
- 10C. Hui, L. Brieger, C. Strohmann, A. P. Antonchick, J. Am. Chem. Soc. 2021, 143, 18864–18870.
- 11
- 11aE. Vitaku, D. T. Smith, J. T. Njardarson, J. Med. Chem. 2014, 57, 10257–10274;
- 11bS. D. Roughley, A. M. Jordan, J. Med. Chem. 2011, 54, 3451–3479;
- 11cJ. S. Carey, D. Laffan, C. Thomson, M. T. Williams, Org. Biomol. Chem. 2006, 4, 2337–2347.
- 12Deposition numbers CCDC 2226734 (for 1 a), CCDC 2191476 (for 3 a), and CCDC 2207235 (for 3 ah) contain the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service.
- 13G. Gilli, V. Bertolasi, F. Bellucci, V. Ferretti, J. Am. Chem. Soc. 1986, 108, 2420–2424.
- 14The yields of byproduct 3 c’, 3 s’, and 3 t’ were 36 %, 39 % and 28 %, respectively. See the Supporting Information for more details.
- 15S. Henness, D. M. Robinson, K. A. Lyseng-Williamson, Drugs 2006, 66, 2109–2119.
- 16
- 16aJ. A. Fernández-Salas, S. Manzini, S. P. Nolan, Chem. Commun. 2013, 49, 9758–9760;
- 16bP.-Q. Huang, W. Ou, F. Han, Chem. Commun. 2016, 52, 11967–11970.
- 17P. Li, X. Xu, J. Chen, H. Yao, A. Lin, Org. Chem. Front. 2018, 5, 1777–1781.
- 18M.-M. Xu, X.-Y. You, Y.-Z. Zhang, Y. Lu, K. Tan, L. Yang, Q. Cai, J. Am. Chem. Soc. 2021, 143, 8993–9001.
- 19T. Ishiyama, M. Murata, N. Miyaura, J. Org. Chem. 1995, 60, 7508–7510.
- 20F. W. Patureau, T. Besset, F. Glorius, Angew. Chem. Int. Ed. 2011, 50, 1064–1067.
- 21C. Hansch, A. Leo, R. W. Taft, Chem. Rev. 1991, 91, 165–195.
- 22
- 22aH. C. Brown, Y. Okamoto, J. Am. Chem. Soc. 1957, 79, 1913–1917;
- 22bT. Mita, M. Sugawara, H. Hasegawa, Y. Sato, J. Org. Chem. 2012, 77, 2159–2168.