Descriptor-Based Volcano Relations Predict Single Atoms for Hydroxylamine Electrosynthesis
Graphical Abstract
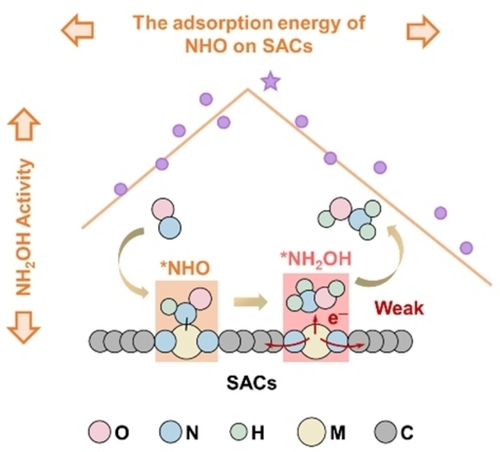
A weak *NH2OH binding affinity and a favorable reaction pathway (*NHO pathway) jointly enable single-atom catalysts (SACs) with superior hydroxylamine (NH2OH) selectivity. Then, an activity volcano plot of Gad(*NHO) is established to predict Mn SACs as optimal electrocatalysts that exhibit pH-dependent activity. Furthermore, Mn−Co geminal-atom catalysts are predicted to optimize Gad(*NHO) and are experimentally proven to enhance NH2OH formation.
Abstract
Hydroxylamine (NH2OH) is an important feedstock in fuels, pharmaceuticals, and agrochemicals. Nanostructured electrocatalysts drive green electrosynthesis of hydroxylamine from nitrogen oxide species in water. However, current electrocatalysts still suffer from low selectivity and manpower-consuming trial-and-error modes, leaving unclear selectivity/activity origins and a lack of catalyst design principles. Herein, we theoretically analyze key determinants of selectivity/activity and propose the adsorption energy of NHO (Gad(*NHO)) as a performance descriptor. A weak *NH2OH binding affinity and a favorable reaction pathway (*NHO pathway) jointly enable single-atom catalysts (SACs) with superior NH2OH selectivity. Then, an activity volcano plot of Gad(*NHO) is established to predict a series of SACs and discover Mn SACs as optimal electrocatalysts that exhibit pH-dependent activity. These theoretical prediction results are also confirmed by experimental results, rationalizing our Gad(*NHO) descriptor. Furthermore, Mn−Co geminal-atom catalysts (GACs) are predicted to optimize Gad(*NHO) and are experimentally proved to enhance NH2OH formation.
Conflict of interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.