Reconstitution of the Final Steps in the Biosynthesis of Valanimycin Reveals the Origin of Its Characteristic Azoxy Moiety
Ziyang Zheng
Department of Chemistry, University of Texas at Austin, Austin, TX-78712 USA
Search for more papers by this authorDr. Jin Xiong
Department of Chemistry, Carnegie Mellon University, Pittsburgh, PA-15213 USA
Search for more papers by this authorDr. Junling Bu
Division of Chemical Biology and Medicinal Chemistry, College of Pharmacy, University of Texas at Austin, Austin, TX-78712 USA
Search for more papers by this authorDaan Ren
Department of Chemistry, University of Texas at Austin, Austin, TX-78712 USA
Search for more papers by this authorYu-Hsuan Lee
Department of Chemistry, University of Texas at Austin, Austin, TX-78712 USA
Search for more papers by this authorDr. Yu-Cheng Yeh
Department of Chemistry, University of Texas at Austin, Austin, TX-78712 USA
Search for more papers by this authorDr. Chia-I Lin
Department of Chemistry, University of Texas at Austin, Austin, TX-78712 USA
Search for more papers by this authorProf. Ronald Parry
Department of Chemistry, Rice University, Houston, TX-77005 USA
Search for more papers by this authorProf. Yisong Guo
Department of Chemistry, Carnegie Mellon University, Pittsburgh, PA-15213 USA
Search for more papers by this authorCorresponding Author
Prof. Hung-wen Liu
Department of Chemistry, University of Texas at Austin, Austin, TX-78712 USA
Division of Chemical Biology and Medicinal Chemistry, College of Pharmacy, University of Texas at Austin, Austin, TX-78712 USA
Search for more papers by this authorZiyang Zheng
Department of Chemistry, University of Texas at Austin, Austin, TX-78712 USA
Search for more papers by this authorDr. Jin Xiong
Department of Chemistry, Carnegie Mellon University, Pittsburgh, PA-15213 USA
Search for more papers by this authorDr. Junling Bu
Division of Chemical Biology and Medicinal Chemistry, College of Pharmacy, University of Texas at Austin, Austin, TX-78712 USA
Search for more papers by this authorDaan Ren
Department of Chemistry, University of Texas at Austin, Austin, TX-78712 USA
Search for more papers by this authorYu-Hsuan Lee
Department of Chemistry, University of Texas at Austin, Austin, TX-78712 USA
Search for more papers by this authorDr. Yu-Cheng Yeh
Department of Chemistry, University of Texas at Austin, Austin, TX-78712 USA
Search for more papers by this authorDr. Chia-I Lin
Department of Chemistry, University of Texas at Austin, Austin, TX-78712 USA
Search for more papers by this authorProf. Ronald Parry
Department of Chemistry, Rice University, Houston, TX-77005 USA
Search for more papers by this authorProf. Yisong Guo
Department of Chemistry, Carnegie Mellon University, Pittsburgh, PA-15213 USA
Search for more papers by this authorCorresponding Author
Prof. Hung-wen Liu
Department of Chemistry, University of Texas at Austin, Austin, TX-78712 USA
Division of Chemical Biology and Medicinal Chemistry, College of Pharmacy, University of Texas at Austin, Austin, TX-78712 USA
Search for more papers by this authorGraphical Abstract
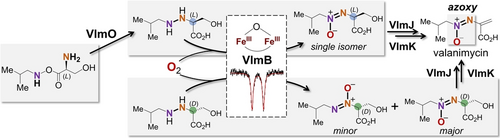
The final steps of valanimycin biosynthesis have been reconstituted in vitro. The oxidation of a dialkyl hydrazine intermediate to the characteristic azoxy moiety in valanimycin is catalyzed by VlmB, which is a non-heme diiron enzyme. The VlmB-catalyzed four-electron oxidation may commence with a resting μ-oxo diferric complex without prior reduction.
Abstract
Valanimycin is an azoxy-containing natural product isolated from the fermentation broth of Streptomyces viridifaciens MG456-hF10. While the biosynthesis of valanimycin has been partially characterized, how the azoxy group is constructed remains obscure. Herein, the membrane protein VlmO and the putative hydrazine synthetase ForJ from the formycin biosynthetic pathway are demonstrated to catalyze N−N bond formation converting O-(l-seryl)-isobutyl hydroxylamine into N-(isobutylamino)-l-serine. Subsequent installation of the azoxy group is shown to be catalyzed by the non-heme diiron enzyme VlmB in a reaction in which the N−N single bond in the VlmO/ForJ product is oxidized by four electrons to yield the azoxy group. The catalytic cycle of VlmB appears to begin with a resting μ-oxo diferric complex in VlmB, as supported by Mössbauer spectroscopy. This study also identifies N-(isobutylamino)-d-serine as an alternative substrate for VlmB leading to two azoxy regioisomers. The reactions catalyzed by the kinase VlmJ and the lyase VlmK during the final steps of valanimycin biosynthesis are established as well. The biosynthesis of valanimycin was thus fully reconstituted in vitro using the enzymes VlmO/ForJ, VlmB, VlmJ and VlmK. Importantly, the VlmB-catalyzed reaction represents the first example of enzyme-catalyzed azoxy formation and is expected to proceed by an atypical mechanism.
Conflict of interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202315844-sup-0001-misc_information.pdf1.6 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1M. Yamato, H. Iinuma, H. Naganawa, Y. Yamagishi, M. Hamada, T. Masuda, H. Umezawa, Y. Abe, M. Hori, J. Antibiot. 1986, 39, 184–191.
- 2M. Yamato, H. Umezawa, N. Sakata, Y. Moriya, M. Hori, J. Antibiot. 1987, 40, 558–560.
- 3M. Wibowo, L. Ding, J. Nat. Prod. 2020, 83, 3482–3491.
- 4Y. Y. Guo, Z. H. Li, T. Y. Xia, Y. L. Du, X. M. Mao, Y. Q. Li, Nat. Commun. 2019, 10, 4420.
- 5
- 5aR. J. Parry, Y. Li, F. L. Lii, J. Am. Chem. Soc. 1992, 114, 10062–10064;
- 5bR. J. Parry, W. Y. Li, Arch. Biochem. Biophys. 1997, 339, 47–54;
- 5cY. Q. Ma, J. Patel, R. J. Parry, Microbiology 2000, 146, 345–352;
- 5dT. Tao, L. B. Alemany, R. J. Parry, Org. Lett. 2003, 5, 1213–1215;
- 5eR. P. Garg, R. J. Parry, Microbiology 2010, 156, 472–483.
- 6R. J. Parry, W. Y. Li, J. Chem. Soc. Chem. Commun. 1994, 995–996.
- 7
- 7aR. J. Parry, W. Y. Li, J. Biol. Chem. 1997, 272, 23303–23311;
- 7bR. J. Parry, W. Y. Li, H. N. Cooper, J. Bacteriol. 1997, 179, 409–416.
- 8R. P. Garg, Y. Q. Ma, J. C. Hoyt, R. J. Parry, Mol. Microbiol. 2002, 46, 505–517.
- 9R. P. Garg, J. M. Gonzalez, R. J. Parry, J. Biol. Chem. 2006, 281, 26785–26791.
- 10R. P. Garg, X. L. Qian, L. B. Alemany, S. Moran, R. J. Parry, Proc. Natl. Acad. Sci. USA 2008, 105, 6543–6547.
- 11R. P. Garg, L. B. Alemany, S. Moran, R. J. Parry, J. Am. Chem. Soc. 2009, 131, 9608–9609.
- 12A. J. Waldman, E. P. Balskus, J. Org. Chem. 2018, 83, 7539–7546.
- 13
- 13aG. L. Ma, H. Candra, L. M. Pang, J. Xiong, Y. Ding, H. T. Tran, Z. J. Low, H. Ye, M. Liu, J. Zheng, M. Fang, B. Cao, Z. X. Liang, J. Am. Chem. Soc. 2022, 144, 1622–1633;
- 13bS. Kawai, A. Yamada, D. Du, Y. Sugai, Y. Katsuyama, Y. Ohnishi, ACS Chem. Biol. 2023, 18, 1821–1828;
- 13cS. Kawai, R. Hagihara, K. Shin-ya, Y. Katsuyama, Y. Ohnishi, Angew. Chem. Int. Ed. 2022, 61, e202211728.
- 14A. Del Rio Flores, F. F. Twigg, Y. Du, W. Cai, D. Q. Aguirre, M. Sato, M. J. Dror, M. Narayanamoorthy, J. Geng, N. A. Zill, R. Zhai, W. Zhang, Nat. Chem. Biol. 2021, 17, 1305–1313.
- 15K. Matsuda, T. Tomita, K. Shin-Ya, T. Wakimoto, T. Kuzuyama, M. Nishiyama, J. Am. Chem. Soc. 2018, 140, 9083–9086.
- 16
- 16aD. Van Cura, T. L. Ng, J. Huang, H. Hager, J. F. Hartwig, J. D. Keasling, E. P. Balskus, Angew. Chem. Int. Ed. 2023, 62, e202304646;
- 16bZ. W. Wei, H. Niikura, M. Wang, K. S. Ryan, Org. Lett. 2023, 25, 4061–4065;
- 16cG. Zhao, S. Yao, K. W. Rothchild, T. Liu, Y. Liu, J. Lian, H. Y. He, K. S. Ryan, Y. L. Du, ChemBioChem 2020, 21, 644–649;
- 16dK. Matsuda, K. Arima, S. Akiyama, Y. Yamada, Y. Abe, H. Suenaga, J. Hashimoto, K. Shin-Ya, M. Nishiyama, T. Wakimoto, J. Am. Chem. Soc. 2022, 144, 12954–12960.
- 17G. Zhao, W. Peng, K. Song, J. Shi, X. Lu, B. Wang, Y. L. Du, Nat. Commun. 2021, 12, 7205.
- 18
- 18aD. Ren, S. A. Wang, Y. Ko, Y. Geng, Y. Ogasawara, H.-w. Liu, Angew. Chem. Int. Ed. 2019, 58, 16512–16516;
- 18bS. A. Wang, Y. Ko, J. Zeng, Y. Geng, D. Ren, Y. Ogasawara, S. Irani, Y. Zhang, H.-w. Liu, J. Am. Chem. Soc. 2019, 141, 6127–6131.
- 19Y. L. Du, H. Y. He, M. A. Higgins, K. S. Ryan, Nat. Chem. Biol. 2017, 13, 836–838.
- 20
- 20aT. L. Ng, R. Rohac, A. J. Mitchell, A. K. Boal, E. P. Balskus, Nature 2019, 566, 94–99;
- 20bH. Y. He, A. C. Henderson, Y. L. Du, K. S. Ryan, J. Am. Chem. Soc. 2019, 141, 4026–4033.
- 21S. Kawai, Y. Sugaya, R. Hagihara, H. Tomita, Y. Katsuyama, Y. Ohnishi, Angew. Chem. Int. Ed. 2021, 60, 10319–10325.
- 22W. S. Mak, X. Wang, R. Arenas, Y. Cui, S. Bertolani, W. Q. Deng, I. Tagkopoulos, D. K. Wilson, J. B. Siegel, Biochemistry 2020, 59, 3834–3843.
- 23
- 23aR. Winkler, C. Hertweck, Angew. Chem. Int. Ed. 2005, 44, 4083–4087;
- 23bM. Simurdiak, J. Lee, H. Zhao, ChemBioChem 2006, 7, 1169–1172;
- 23cY. S. Choi, H. Zhang, J. S. Brunzelle, S. K. Nair, H. Zhao, Proc. Natl. Acad. Sci. USA. 2008, 105, 6858–6863.
- 24
- 24aH. Lu, E. Chanco, H. Zhao, Tetrahedron 2012, 68, 7651–7654;
- 24bC. J. Knoot, E. G. Kovaleva, J. D. Lipscomb, J. Biol. Inorg. Chem. 2016, 21, 589–603.
- 25C. A. Brown, G. J. Remar, R. L. Musselman, E. I. Solomon, Inorg. Chem. 1995, 34, 688–717.
- 26V. K. Korboukh, N. Li, E. W. Barr, J. M. Bollinger, C. Krebs, J. Am. Chem. Soc. 2009, 131, 13608–13609.
- 27R. Bhushan, H. Bruckner, Amino Acids 2004, 27, 231–247.
- 28
- 28aA. J. Jasniewski, L. Que, Chem. Rev. 2018, 118, 2554–2592;
- 28bL. J. Rajakovich, B. Zhang, M. J. McBride, A. K. Boal, C. Krebs, J. M. Bollinger in Comprehensive Natural Products III (Eds.: H.-w. Liu, T. P. Begley) Elsevier, Oxford, 2020, pp 215–250.
10.1016/B978-0-12-409547-2.14864-4 Google Scholar
- 29
- 29aR. Fu, R. Gupta, J. Geng, K. Dornevil, S. Wang, Y. Zhang, M. P. Hendrich, A. Liu, J. Biol. Chem. 2011, 286, 26541–26554;
- 29bY. Wang, I. Davis, Y. Chan, S. G. Naik, W. P. Griffith, A. Liu, J. Biol. Chem. 2020, 295, 11789–11802.
- 30
- 30aO. M. Manley, R. Fan, Y. Guo, T. M. Makris, J. Am. Chem. Soc. 2019, 141, 8684–8688;
- 30bB. Zhang, L. J. Rajakovich, D. Van Cura, E. J. Blaesi, A. J. Mitchell, C. R. Tysoe, X. Zhu, B. R. Streit, Z. Rui, W. Zhang, A. K. Boal, C. Krebs, J. M. Bollinger, J. Am. Chem. Soc. 2019, 141, 14510–14514;
- 30cO. M. Manley, H. Tang, S. Xue, Y. Guo, W. C. Chang, T. M. Makris, J. Am. Chem. Soc. 2021, 143, 21416–21424;
- 30dM. J. McBride, M. A. Nair, D. Sil, J. W. Slater, M. E. Neugebauer, M. C. Y. Chang, A. K. Boal, C. Krebs, J. M. Bollinger, Biochemistry 2022, 61, 689–702.
- 31
- 31aN. Li, V. K. Korboukh, C. Krebs, J. M. Bollinger, Proc. Natl. Acad. Sci. USA 2010, 107, 15722–15727;
- 31bK. Park, N. Li, Y. Kwak, M. Srnec, C. B. Bell, L. V. Liu, S. D. Wong, Y. Yoda, S. Kitao, M. Seto, M. Hu, J. Zhao, C. Krebs, J. M. Bollinger, E. I. Solomon, J. Am. Chem. Soc. 2017, 139, 7062–7070.
- 32
- 32aA. J. Komor, B. S. Rivard, R. Fan, Y. Guo, L. Que, J. D. Lipscomb, J. Am. Chem. Soc. 2016, 138, 7411–7421;
- 32bA. J. Jasniewski, A. J. Komor, J. D. Lipscomb, L. Que, J. Am. Chem. Soc. 2017, 139, 10472–10485.
- 33A. J. Komor, B. S. Rivard, R. Fan, Y. Guo, L. Que, J. D. Lipscomb, Biochemistry 2017, 56, 4940–4950.