Overlooked Formation of Carbonate Radical Anions in the Oxidation of Iron(II) by Oxygen in the Presence of Bicarbonate
Graphical Abstract
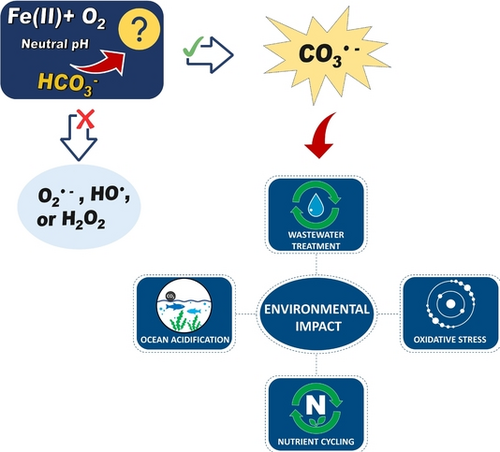
The rapid generation of carbonate radical anions (CO3.−) in the oxidation of FeII by O2 in the presence of bicarbonate (HCO3−) is shown. The results highlight the importance of considering the formation of CO3.− in the geochemical cycling of iron and carbon and show that the presence of bicarbonate dramatically changes the mechanism and kinetics of the reaction of Fe(H2O)62++O2 under physiological and environmental conditions.
Abstract
Iron(II), (Fe(H2O)62+, (FeII) participates in many reactions of natural and biological importance. It is critically important to understand the rates and the mechanism of FeII oxidation by dissolved molecular oxygen, O2, under environmental conditions containing bicarbonate (HCO3−), which exists up to millimolar concentrations. In the absence and presence of HCO3−, the formation of reactive oxygen species (O2⋅−, H2O2, and HO⋅) in FeII oxidation by O2 has been suggested. In contrast, our study demonstrates for the first time the rapid generation of carbonate radical anions (CO3⋅−) in the oxidation of FeII by O2 in the presence of bicarbonate, HCO3−. The rate of the formation of CO3⋅− may be expressed as d[CO3⋅−]/dt=[FeII[[O2][HCO3−]2. The formation of reactive species was investigated using 1H nuclear magnetic resonance (1H NMR) and gas chromatographic techniques. The study presented herein provides new insights into the reaction mechanism of FeII oxidation by O2 in the presence of bicarbonate and highlights the importance of considering the formation of CO3⋅− in the geochemical cycling of iron and carbon.
Open Research
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.