Asymmetric Dearomatization of Phenols via Ligand-Enabled Cooperative Gold Catalysis
Dr. Yongliang Zhang
Department of Chemistry and Biochemistry, University of California, Santa Barbara, CA, 93106 USA
These authors contributed equally to this work.
Search for more papers by this authorKe Zhao
Department of Chemistry and Biochemistry, University of California, Santa Barbara, CA, 93106 USA
These authors contributed equally to this work.
Search for more papers by this authorXinyi Li
Department of Chemistry and Biochemistry, University of California, Santa Barbara, CA, 93106 USA
Search for more papers by this authorCarlos D. Quintanilla
Department of Chemistry and Biochemistry, University of California, Santa Barbara, CA, 93106 USA
Search for more papers by this authorCorresponding Author
Prof. Dr. Liming Zhang
Department of Chemistry and Biochemistry, University of California, Santa Barbara, CA, 93106 USA
Search for more papers by this authorDr. Yongliang Zhang
Department of Chemistry and Biochemistry, University of California, Santa Barbara, CA, 93106 USA
These authors contributed equally to this work.
Search for more papers by this authorKe Zhao
Department of Chemistry and Biochemistry, University of California, Santa Barbara, CA, 93106 USA
These authors contributed equally to this work.
Search for more papers by this authorXinyi Li
Department of Chemistry and Biochemistry, University of California, Santa Barbara, CA, 93106 USA
Search for more papers by this authorCarlos D. Quintanilla
Department of Chemistry and Biochemistry, University of California, Santa Barbara, CA, 93106 USA
Search for more papers by this authorCorresponding Author
Prof. Dr. Liming Zhang
Department of Chemistry and Biochemistry, University of California, Santa Barbara, CA, 93106 USA
Search for more papers by this authorGraphical Abstract
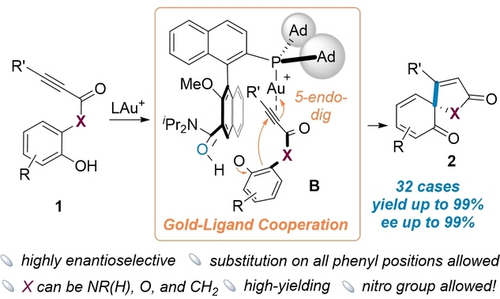
Asymmetric dearomatization of phenols was achieved by employing chiral bifunctional phosphine ligands in cooperative gold catalysis. This transformation demonstrated a remarkable generality, affording the desired product with high yields (up to 99 %) and excellent enantioselectivities (ee up to 99 %).
Abstract
By employing a chiral bifunctional phosphine ligand, a gold(I)-catalyzed efficient and highly enantioselective dearomatization of phenols is achieved via versatile metal-ligand cooperation. The reaction is proven to be remarkably general in scope, permitting substitutions at all four remaining benzene positions, accommodating electron-withdrawing groups including strongly deactivating nitro, and allowing carbon-based groups of varying steric bulk including tert-butyl at the alkyne terminus. Moreover, besides N-(o-hydroxyphenyl)alkynamides, the corresponding ynoates and ynones are all suitable substrates. Spirocyclohexadienone-pyrrol-2-ones, spirocyclohexadienone-butenolides, and spirocyclohexadenone-cyclopentenones are formed in yields up to 99 % and with ee up to 99 %.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202309256-sup-0001-crystal_structure_combined.cif821 KB | Supporting Information |
| anie202309256-sup-0001-misc_information.pdf45.4 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1C. Zheng, S. L. You, ACS Cent. Sci. 2021, 7, 432–444.
- 2Y. Z. Cheng, Z. Feng, X. Zhang, S. L. You, Chem. Soc. Rev. 2022, 51, 2145–2170.
- 3L. Pouységu, D. Deffieux, S. Quideau, Tetrahedron 2010, 66, 2235–2261.
- 4S. P. Roche, J. A. Porco Jr., Angew. Chem. Int. Ed. 2011, 50, 4068–4093.
- 5C. Zheng, S. L. You, Nat. Prod. Rep. 2019, 36, 1589–1605.
- 6F. Lovering, J. Bikker, C. Humblet, J. Med. Chem. 2009, 52, 6752–6756.
- 7W. Sun, G. Li, L. Hong, R. Wang, Org. Biomol. Chem. 2016, 14, 2164–2176.
- 8W. T. Wu, L. Zhang, S. L. You, Chem. Soc. Rev. 2016, 45, 1570–1580.
- 9S. Quideau, L. Pouységu, D. Deffieux, Synlett 2008, 2008, 467–495.
- 10T. Nemoto, Y. Ishige, M. Yoshida, Y. Kohno, M. Kanematsu, Y. Hamada, Org. Lett. 2010, 12, 5020–5023.
- 11L. Yang, H. Zheng, L. Luo, J. Nan, J. Liu, Y. Wang, X. Luan, J. Am. Chem. Soc. 2015, 137, 4876–4879.
- 12E. Lee, Y. Hwang, Y. B. Kim, D. Kim, S. Chang, J. Am. Chem. Soc. 2021, 143, 6363–6369.
- 13R. Q. Xu, Q. Gu, W. T. Wu, Z. A. Zhao, S. L. You, J. Am. Chem. Soc. 2014, 136, 15469–15472.
- 14S. Rousseaux, J. García-Fortanet, M. A. Del Aguila Sanchez, S. L. Buchwald, J. Am. Chem. Soc. 2011, 133, 9282–9285.
- 15R. J. Phipps, F. D. Toste, J. Am. Chem. Soc. 2013, 135, 1268–1271.
- 16M. Uyanik, T. Yasui, K. Ishihara, Angew. Chem. Int. Ed. 2013, 52, 9215–9218.
- 17C. Bosset, R. Coffinier, P. A. Peixoto, M. El Assal, K. Miqueu, J. M. Sotiropoulos, L. Pouysegu, S. Quideau, Angew. Chem. Int. Ed. 2014, 53, 9860–9864.
- 18S. A. Baker Dockrey, A. L. Lukowski, M. R. Becker, A. R. H. Narayan, Nat. Chem. 2018, 10, 119–125.
- 19J. Zhu, N. P. Grigoriadis, J. P. Lee, J. A. Porco, J. Am. Chem. Soc. 2005, 127, 9342–9343.
- 20T. D. D'Arcy, M. R. J. Elsegood, B. R. Buckley, Angew. Chem. Int. Ed. 2022, 61, e202205278.
- 21S. Dong, J. Zhu, J. A. Porco Jr., J. Am. Chem. Soc. 2008, 130, 2738–2739.
- 22K. Du, P. Guo, Y. Chen, Z. Cao, Z. Wang, W. Tang, Angew. Chem. Int. Ed. 2015, 54, 3033–3037.
- 23W.-D. Chu, T.-T. Liang, H. Ni, Z.-H. Dong, Z. Shao, Y. Liu, C.-Y. He, R. Bai, Q.-Z. Liu, Org. Lett. 2022, 24, 4865–4870.
- 24L. Shao, X.-P. Hu, Chem. Commun. 2017, 53, 8192–8195.
- 25X. Cheng, L. Zhang, CCS Chem. 2021, 3, 1989–2002.
- 26Y. Wang, Z. Wang, Y. Li, G. Wu, Z. Cao, L. Zhang, Nat. Commun. 2014, 5, 3470.
- 27Z. Wang, C. Nicolini, C. Hervieu, Y. F. Wong, G. Zanoni, L. Zhang, J. Am. Chem. Soc. 2017, 139, 16064–16067.
- 28K. Zhao, P. Kohnke, Z. Yang, X. Cheng, S. L. You, L. Zhang, Angew. Chem. Int. Ed. 2022, 61, e202207518.
- 29A. A. Nechaev, K. Van Hecke, M. Zaman, S. Kashtanov, L. Ungur, O. P. Pereshivko, V. A. Peshkov, E. V. Van der Eycken, J. Org. Chem. 2018, 83, 8170–8182.
- 30Y. He, D. Wu, Z. Li, K. Robeyns, L. Van Meervelt, E. V. Van der Eycken, Org. Biomol. Chem. 2019, 17, 6284–6292.
- 31Y. He, Z. Liu, D. Wu, Z. Li, K. Robeyns, L. Van Meervelt, E. V. Van der Eycken, Org. Lett. 2019, 21, 4469–4474.
- 32T. Okitsu, D. Nakazawa, A. Kobayashi, M. Mizohata, Y. In, T. Ishida, A. Wada, Synlett 2010, 203–206.
- 33H. Sahoo, G. S. Grandhi, I. Ramakrishna, M. Baidya, Org. Biomol. Chem. 2019, 17, 10163–10166.
- 34A. M. Nair, I. Halder, S. Khan, C. M. R. Volla, Adv. Synth. Catal. 2020, 362, 224–229.
- 35A. M. S. Recchi, P. H. P. Rosa, D. F. Back, G. Zeni, Org. Biomol. Chem. 2020, 18, 3544–3551.
- 36X. Y. Liu, Y. Qin, Nat. Prod. Rep. 2017, 34, 1044–1050.
- 37L. Liu, Y. Han, J. Xiao, L. Li, L. Guo, X. Jiang, L. Kong, Y. Che, J. Nat. Prod. 2016, 79, 2616–2623.
- 38C. C. Liao, R. K. Peddinti, Acc. Chem. Res. 2002, 35, 856–866.
- 39Deposition numbers 2258971 (2 v), 2258972 (2 y), and 2258973 (5) contain the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service.
- 40S. R. Sahoo, B. Das, D. Sarkar, F. Henkel, H. Reuter, Eur. J. Org. Chem. 2020, 891–896.
- 41S. Tcyrulnikov, J. M. Curto, P. H. Gilmartin, M. C. Kozlowski, J. Org. Chem. 2018, 83, 12207–12212.
- 42A. K. Clarke, J. T. R. Liddon, J. D. Cuthbertson, R. J. K. Taylor, W. P. Unsworth, Org. Biomol. Chem. 2017, 15, 233–245.
- 43A. K. Clarke, M. J. James, P. O'Brien, R. J. K. Taylor, W. P. Unsworth, Angew. Chem. Int. Ed. 2016, 55, 13798–13802.