A Radical Pathway and Stabilized Li Anode Enabled by Halide Quaternary Ammonium Electrolyte Additives for Lithium-Sulfur Batteries
Graphical Abstract
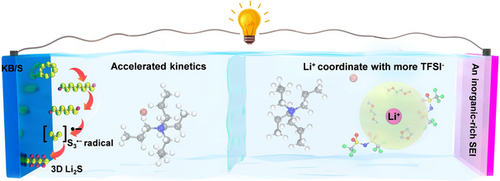
The tetrapropylammonium bromide (T3Br) additive in a Li−S electrolyte can trigger the S3.− radical pathway, which not only enhances the sulfur redox kinetics but also results in a 3D Li2S nucleation. In addition, the T3Br additive weakens the solvation structure of Li+ and creates a LiF-rich solid electrolyte interface on Li anode.
Abstract
Passivation of the sulfur cathode by insulating lithium sulfide restricts the reversibility and sulfur utilization of Li−S batteries. 3D nucleation of Li2S enabled by radical conversion may significantly boost the redox kinetics. Electrolytes with high donor number (DN) solvents allow for tri-sulfur (S3⋅−) radicals as intermediates, however, the catastrophic reactivity of such solvents with Li anodes pose a great challenge for their practical application. Here, we propose the use of quaternary ammonium salts as electrolyte additives, which can preserve the partial high-DN characteristics that trigger the S3⋅− radical pathway, and inhibit the growth of Li dendrites. Li−S batteries with tetrapropylammonium bromide (T3Br) electrolyte additive deliver the outstanding cycling stability (700 cycles at 1 C with a low-capacity decay rate of 0.049 % per cycle), and high capacity under a lean electrolyte of 5 μLelectrolyte mgsulfur−1. This work opens a new avenue for the development of electrolyte additives for Li−S batteries.
Conflict of interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.