Anion-tethered Single Lithium-ion Conducting Polyelectrolytes through UV-induced Free Radical Polymerization for Improved Morphological Stability of Lithium Metal Anodes
Graphical Abstract
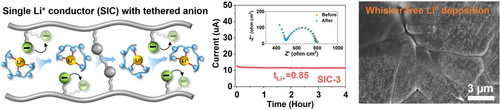
The ion transport kinetics, Li0 deposition behavior, and electrochemical performances of a dual-ion conducting polymer electrolyte and a single-ion conducting polyelectrolyte (SIC) were investigated. The SIC electrolyte showed improved Li+ transference number (0.85), uniform Li0 morphology, enhanced critical current density (2.4 mA/cm2), and durability of 4500 cycles in Li0-LiFePO4 battery.
Abstract
Single Li+ ion conducting polyelectrolytes (SICs), which feature covalently tethered counter-anions along their backbone, have the potential to mitigate dendrite formation by reducing concentration polarization and preventing salt depletion. However, due to their low ionic conductivity and complicated synthetic procedure, the successful validation of these claimed advantages in lithium metal (Li0) anode batteries remains limited. In this study, we fabricated a SIC electrolyte using a single-step UV polymerization approach. The resulting electrolyte exhibited a high Li+ transference number (t+) of 0.85 and demonstrated good Li+ conductivity (6.3×10−5 S/cm at room temperature), which is comparable to that of a benchmark dual ion conductor (DIC, 9.1×10−5 S/cm). Benefitting from the high transference number of SIC, it displayed a three-fold higher critical current density (2.4 mA/cm2) compared to DIC (0.8 mA/cm2) by successfully suppressing concentration polarization-induced short-circuiting. Additionally, the t+ significantly influenced the deposition behavior of Li0, with SIC yielding a uniform, compact, and mosaic-like morphology, while the low t+ DIC resulted in a porous morphology with Li0 whiskers. Using the SIC electrolyte, Li0||LiFePO4 cells exhibited stable operation for 4500 cycles with 70.5 % capacity retention at 22 °C.
Conflict of interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.