Tracking an FeV(O) Intermediate for Water Oxidation in Water
Xiang-Zhu Wei
Key Laboratory of Photochemical Conversion and Optoelectronic Materials, New Cornerstone Science Laboratory, Technical Institute of Physics and Chemistry, Chinese Academy of Sciences, Beijing, 100190 P. R. China
School of Future Technology, University of Chinese Academy of Sciences, Beijing, 100049 P. R. China
Search for more papers by this authorTian-Yu Ding
Key Laboratory of Photochemical Conversion and Optoelectronic Materials, New Cornerstone Science Laboratory, Technical Institute of Physics and Chemistry, Chinese Academy of Sciences, Beijing, 100190 P. R. China
School of Future Technology, University of Chinese Academy of Sciences, Beijing, 100049 P. R. China
Search for more papers by this authorDr. Yang Wang
Key Laboratory of Photochemical Conversion and Optoelectronic Materials, New Cornerstone Science Laboratory, Technical Institute of Physics and Chemistry, Chinese Academy of Sciences, Beijing, 100190 P. R. China
School of Future Technology, University of Chinese Academy of Sciences, Beijing, 100049 P. R. China
Search for more papers by this authorDr. Bing Yang
Key Laboratory of Photochemical Conversion and Optoelectronic Materials, New Cornerstone Science Laboratory, Technical Institute of Physics and Chemistry, Chinese Academy of Sciences, Beijing, 100190 P. R. China
School of Future Technology, University of Chinese Academy of Sciences, Beijing, 100049 P. R. China
Search for more papers by this authorDr. Qing-Qing Yang
Key Laboratory of Photochemical Conversion and Optoelectronic Materials, New Cornerstone Science Laboratory, Technical Institute of Physics and Chemistry, Chinese Academy of Sciences, Beijing, 100190 P. R. China
School of Future Technology, University of Chinese Academy of Sciences, Beijing, 100049 P. R. China
Search for more papers by this authorCorresponding Author
Prof. Dr. Shengfa Ye
State Key Laboratory of Catalysis, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian, 116023 P. R. China
Search for more papers by this authorProf. Dr. Chen-Ho Tung
Key Laboratory of Photochemical Conversion and Optoelectronic Materials, New Cornerstone Science Laboratory, Technical Institute of Physics and Chemistry, Chinese Academy of Sciences, Beijing, 100190 P. R. China
School of Future Technology, University of Chinese Academy of Sciences, Beijing, 100049 P. R. China
Search for more papers by this authorCorresponding Author
Prof. Dr. Li-Zhu Wu
Key Laboratory of Photochemical Conversion and Optoelectronic Materials, New Cornerstone Science Laboratory, Technical Institute of Physics and Chemistry, Chinese Academy of Sciences, Beijing, 100190 P. R. China
School of Future Technology, University of Chinese Academy of Sciences, Beijing, 100049 P. R. China
Search for more papers by this authorXiang-Zhu Wei
Key Laboratory of Photochemical Conversion and Optoelectronic Materials, New Cornerstone Science Laboratory, Technical Institute of Physics and Chemistry, Chinese Academy of Sciences, Beijing, 100190 P. R. China
School of Future Technology, University of Chinese Academy of Sciences, Beijing, 100049 P. R. China
Search for more papers by this authorTian-Yu Ding
Key Laboratory of Photochemical Conversion and Optoelectronic Materials, New Cornerstone Science Laboratory, Technical Institute of Physics and Chemistry, Chinese Academy of Sciences, Beijing, 100190 P. R. China
School of Future Technology, University of Chinese Academy of Sciences, Beijing, 100049 P. R. China
Search for more papers by this authorDr. Yang Wang
Key Laboratory of Photochemical Conversion and Optoelectronic Materials, New Cornerstone Science Laboratory, Technical Institute of Physics and Chemistry, Chinese Academy of Sciences, Beijing, 100190 P. R. China
School of Future Technology, University of Chinese Academy of Sciences, Beijing, 100049 P. R. China
Search for more papers by this authorDr. Bing Yang
Key Laboratory of Photochemical Conversion and Optoelectronic Materials, New Cornerstone Science Laboratory, Technical Institute of Physics and Chemistry, Chinese Academy of Sciences, Beijing, 100190 P. R. China
School of Future Technology, University of Chinese Academy of Sciences, Beijing, 100049 P. R. China
Search for more papers by this authorDr. Qing-Qing Yang
Key Laboratory of Photochemical Conversion and Optoelectronic Materials, New Cornerstone Science Laboratory, Technical Institute of Physics and Chemistry, Chinese Academy of Sciences, Beijing, 100190 P. R. China
School of Future Technology, University of Chinese Academy of Sciences, Beijing, 100049 P. R. China
Search for more papers by this authorCorresponding Author
Prof. Dr. Shengfa Ye
State Key Laboratory of Catalysis, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian, 116023 P. R. China
Search for more papers by this authorProf. Dr. Chen-Ho Tung
Key Laboratory of Photochemical Conversion and Optoelectronic Materials, New Cornerstone Science Laboratory, Technical Institute of Physics and Chemistry, Chinese Academy of Sciences, Beijing, 100190 P. R. China
School of Future Technology, University of Chinese Academy of Sciences, Beijing, 100049 P. R. China
Search for more papers by this authorCorresponding Author
Prof. Dr. Li-Zhu Wu
Key Laboratory of Photochemical Conversion and Optoelectronic Materials, New Cornerstone Science Laboratory, Technical Institute of Physics and Chemistry, Chinese Academy of Sciences, Beijing, 100190 P. R. China
School of Future Technology, University of Chinese Academy of Sciences, Beijing, 100049 P. R. China
Search for more papers by this authorGraphical Abstract
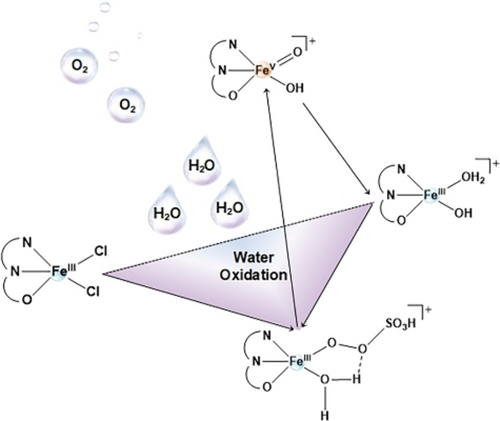
An electron-rich and oxidation-resistant ligand is effective at stabilizing high-valent iron-oxo intermediates involved in the water oxidation reaction. UV/Vis, XAS, EPR, electrochemical and kinetic measurements, allow a high-valent FeV(O) species that participates in O−O bond formation with higher reactivity and shorter lifetime to be tracked in a real catalytic water oxidation reaction.
Abstract
High-valent iron-oxo species are appealing for conducting O−O bond formation for water oxidation reactions. However, their high reactivity poses a great challenge to the dissection of their chemical transformations. Herein, we introduce an electron-rich and oxidation-resistant ligand, 2-[(2,2′-bipyridin)-6-yl]propan-2-ol to stabilize such fleeting intermediates. Advanced spectroscopies and electrochemical studies demonstrate a high-valent FeV(O) species formation in water. Combining kinetic and oxygen isotope labelling experiments and organic reactions indicates that the FeV(O) species is responsible for O−O bond formation via water nucleophilic attack under the real catalytic water oxidation conditions.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202308192-sup-0001-1.cif307.1 KB | Supporting Information |
| anie202308192-sup-0001-2.cif367.1 KB | Supporting Information |
| anie202308192-sup-0001-3.cif365.2 KB | Supporting Information |
| anie202308192-sup-0001-misc_information.pdf1.5 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aM. Yagi, M. Kaneko, Chem. Rev. 2001, 101, 21–36;
- 1bM. D. Kärkäs, O. Verho, E. V. Johnston, B. Akermark, Chem. Rev. 2014, 114, 11863–12001;
- 1cJ. D. Blakemore, R. H. Crabtree, G. W. Brudvig, Chem. Rev. 2015, 115, 12974–13005;
- 1dJ. Lin, Q. Han, Y. Ding, Chem. Rec. 2018, 18, 1531–1547;
- 1eL. H. Zhang, S. Mathew, J. Hessels, J. N. H. Reek, F. Yu, ChemSusChem 2021, 14, 234–250;
- 1fX. Jiang, J. Li, B. Yang, X.-Z. Wei, B.-W. Dong, Y. Kao, M.-Y. Huang, C.-H. Tung, L.-Z. Wu, Angew. Chem. Int. Ed. 2018, 57, 7850–7854;
- 1gX. Jiang, B. Yang, Q.-Q. Yang, C.-H. Tung, L.-Z. Wu, Chem. Commun. 2018, 54, 4794–4797;
- 1hH.-Y. Du, S.-C. Chen, X.-J. Su, L. Jiao, M.-T. Zhang, J. Am. Chem. Soc. 2018, 140, 1557–1565;
- 1iX-J. Su, M. Gao, L. Jiao, R.-Z. Liao, P. E. Siegbahn, J.-P. Cheng, M.-T. Zhang, Angew. Chem. Int. Ed. 2015, 54, 4909–4914.
- 2
- 2aS. W. Gersten, G. J. Samuels, T. J. Meyer, J. Am. Chem. Soc. 1982, 104, 4029–4030;
- 2bL. Duan, F. Bozoglian, S. Mandal, B. Stewart, T. Privalov, A. Llobet, L. Sun, Nat. Chem. 2012, 4, 418–423;
- 2cR. Matheu, M. Z. Ertem, C. Gimbert-Surinach, X. Sala, A. Llobet, Chem. Rev. 2019, 119, 3453–3471;
- 2dB. Zhang, L. Sun, J. Am. Chem. Soc. 2019, 141, 5565–5580;
- 2eB. Yang, X. Jiang, Q. Guo, T. Lei, L.-P. Zhang, B. Chen, C.-H. Tung, L.-Z. Wu, Angew. Chem. Int. Ed. 2016, 55, 6229–6234;
- 2fQ.-Q. Yang, X. Jiang, B. Yang, Y. Wang, C.-H. Tung, L.-Z. Wu, iScience 2020, 23, 100969.
- 3
- 3aN. D. McDaniel, F. J. Coughlin, L. L. Tinker, S. Bernhard, J. Am. Chem. Soc. 2008, 130, 210–217;
- 3bJ. F. Hull, D. Balcells, J. D. Blakemore, C. D. Incarvito, O. Eisenstein, G. W. Brudvig, R. H. Crabtree, J. Am. Chem. Soc. 2009, 131, 8730–8731;
- 3cD. B. Grotjahn, D. B. Brown, J. K. Martin, D. C. Marelius, M. C. Abadjian, H. N. Tran, G. Kalyuzhny, K. S. Vecchio, Z. G. Specht, S. A. Cortes-Llamas, V. Miranda-Soto, C. van Niekerk, C. E. Moore, A. L. Rheingold, J. Am. Chem. Soc. 2011, 133, 19024–19027;
- 3dB. van Dijk, G. M. Rodriguez, L. Wu, J. P. Hofmann, A. Macchioni, D. G. H. Hetterscheid, ACS Catal. 2020, 10, 4398–4410;
- 3eA. Volpe, M. Natali, C. Graiff, A. Sartorel, C. Tubaro, M. Bonchio, Dalton Trans. 2020, 49, 2696–2705;
- 3fZ. Mazloomi, J. Margalef, M. Gil-Sepulcre, N. Romero, M. Albrecht, A. Llobet, X. Sala, O. Pàmies, M. Diéguez, Inorg. Chem. 2020, 59, 12337–12347.
- 4W. C. Ellis, N. D. McDaniel, S. Bernhard, T. J. Collins, J. Am. Chem. Soc. 2010, 132, 10990–10991.
- 5C. Panda, J. Debgupta, D. Diaz Diaz, K. K. Singh, S. Sen Gupta, B. B. Dhar, J. Am. Chem. Soc. 2014, 136, 12273–12282.
- 6J. L. Fillol, Z. Codola, I. Garcia-Bosch, L. Gomez, J. J. Pla, M. Costas, Nat. Chem. 2011, 3, 807–813.
- 7R. Ezhov, A. K. Ravari, Y. Pushkar, Angew. Chem. Int. Ed. 2020, 59, 13502–13505.
- 8
- 8aS. W. Sheehan, J. M. Thomsen, U. Hintermair, R. H. Crabtree, G. W. Brudvig, C. A. Schmuttenmaer, Nat. Commun. 2015, 6, 6469–6478;
- 8bT. K. Michaelos, H. M. C. Lant, L. S. Sharninghausen, S. M. Craig, F. S. Menges, B. Q. Mercado, G. W. Brudvig, R. H. Crabtree, ChemPlusChem 2016, 81, 1129–1132;
- 8cD. L. Huang, R. Beltran-Suito, J. M. Thomsen, S. M. Hashmi, K. L. Materna, S. W. Sheehan, B. Q. Mercado, G. W. Brudvig, R. H. Crabtree, Inorg. Chem. 2016, 55, 2427–2435;
- 8dT. K. Michaelos, D. Y. Shopov, S. B. Sinha, L. S. Sharninghausen, K. J. Fisher, H. M. C. Lant, R. H. Crabtree, G. W. Brudvig, Acc. Chem. Res. 2017, 50, 952–959.
- 9S. I. Shylin, M. V. Pavliuk, L. D′Amario, F. Mamedov, J. Sa, G. Berggren, I. O. Fritsky, Chem. Commun. 2019, 55, 3335–3338.
- 10M. K. Coggins, M.-T. Zhang, A. K. Vannucci, C. J. Dares, T. J. Meyer, J. Am. Chem. Soc. 2014, 136, 5531–5534.
- 11J. Schneider, R. E. Bangle, W. B. Swords, L. Troian-Gautier, G. J. Meyer, J. Am. Chem. Soc. 2019, 141, 9758–9763.
- 12R. Fan, J. Serrano-Plana, W. N. Oloo, A. Draksharapu, E. Delgado-Pinar, A. Company, V. Martin-Diaconescu, M. Borrell, J. Lloret-Fillol, E. Garcia-Espana, Y. Guo, E. L. Bominaar, L. Que Jr., M. Costas, E. Munck, J. Am. Chem. Soc. 2018, 140, 3916–3928.
- 13S. Pattanayak, A. J. Jasniewski, A. Rana, A. Draksharapu, K. K. Singh, A. Weitz, M. Hendrich, L. Que Jr., A. Dey, S. Sen Gupta, Inorg. Chem. 2017, 56, 6352–6361.
- 14
- 14aH. C. Chang, B. Mondal, H. Fang, F. Neese, E. Bill, S. Ye, J. Am. Chem. Soc. 2019, 141, 2421–2434;
- 14bF. Tiago de Oliveira, A. Chanda, D. Banerjee, X. Shan, S. Mondal, L. Que Jr., E. L. Bominaar, E. Munck, T. J. Collins, Science 2007, 315, 835–838;
- 14cA. M. Zima, O. Y. Lyakin, K. P. Bryliakov, E. P. Talsi, ChemCatChem 2019, 11, 5345–5352;
- 14dO. Y. Lyakin, K. P. Bryliakov, G. J. Britovsek, E. P. Talsi, J. Am. Chem. Soc. 2009, 131, 10798–10799.
- 15
- 15aA. R. Parent, R. H. Crabtree, G. W. Brudvig, Chem. Soc. Rev. 2013, 42, 2247–2252;
- 15bJ. Limburg, J. S. Vrettos, H. Chen, J. C. de Paula, R. H. Crabtree, G. W. Brudvig, J. Am. Chem. Soc. 2001, 123, 423–430.
- 16S. Kal, S. Xu, L. Que Jr., Angew. Chem. Int. Ed. 2020, 59, 7332–7349.
- 17J. Bernadou, B. Meunier, Chem. Commun. 1998, 2167–2173.
- 18V. A. Larson, B. Battistella, K. Ray, N. Lehnert, W. Nam, Nat. Chem. Rev. 2020, 4, 404–419.
- 19Deposition numbers 2075532 (for Fe(bipy-alk)Cl2), 2244611 (for Zn(bipy-AlkH)Cl2), 2244610 (for Fe(bipy-alk)(NO3)2) contain the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service.
- 20Tracking FeV(O) species in situ is challenging, especially in water. [FeV=O(OH)(Pytacn)]2+, one of the most representative catalysts in organic solvent could be detected by HRMS. However in water, [FeIV(O)(OH2)(Pytacn)]2+ was found instead. Most iron complexes readily undergo hydrolysis, and thus cannot maintain their molecular structures, especially in strong oxidizing conditions. Furthermore, water that accelerates the decay of FeV(O) complexes greatly reduces their concentrations and hence lowers the probability to detect and characterize in situ such fleeting intermediates, especially in a real catalytic condition.