High-Rate CO2 Electrolysis to Formic Acid over a Wide Potential Window: An Electrocatalyst Comprised of Indium Nanoparticles on Chitosan-Derived Graphene
Jiahui Bi
Beijing National Laboratory for Molecular Sciences, CAS Key Laboratory of Colloid, Interface and Chemical Thermodynamics, CAS Research/Education Center for Excellence in Molecular Sciences, Institute of Chemistry, Chinese Academy of Sciences, Beijing, 100190 P. R. China
School of Chemistry and Chemical Engineering, University of Chinese Academy of Sciences, Beijing, 100049 P. R. China
Search for more papers by this authorPengsong Li
Beijing National Laboratory for Molecular Sciences, CAS Key Laboratory of Colloid, Interface and Chemical Thermodynamics, CAS Research/Education Center for Excellence in Molecular Sciences, Institute of Chemistry, Chinese Academy of Sciences, Beijing, 100190 P. R. China
School of Chemistry and Chemical Engineering, University of Chinese Academy of Sciences, Beijing, 100049 P. R. China
Search for more papers by this authorJiyuan Liu
Beijing National Laboratory for Molecular Sciences, CAS Key Laboratory of Colloid, Interface and Chemical Thermodynamics, CAS Research/Education Center for Excellence in Molecular Sciences, Institute of Chemistry, Chinese Academy of Sciences, Beijing, 100190 P. R. China
School of Chemistry and Chemical Engineering, University of Chinese Academy of Sciences, Beijing, 100049 P. R. China
Search for more papers by this authorYong Wang
Beijing National Laboratory for Molecular Sciences, CAS Key Laboratory of Colloid, Interface and Chemical Thermodynamics, CAS Research/Education Center for Excellence in Molecular Sciences, Institute of Chemistry, Chinese Academy of Sciences, Beijing, 100190 P. R. China
School of Chemistry and Chemical Engineering, University of Chinese Academy of Sciences, Beijing, 100049 P. R. China
Search for more papers by this authorXinning Song
Beijing National Laboratory for Molecular Sciences, CAS Key Laboratory of Colloid, Interface and Chemical Thermodynamics, CAS Research/Education Center for Excellence in Molecular Sciences, Institute of Chemistry, Chinese Academy of Sciences, Beijing, 100190 P. R. China
School of Chemistry and Chemical Engineering, University of Chinese Academy of Sciences, Beijing, 100049 P. R. China
Search for more papers by this authorDr. Xinchen Kang
Beijing National Laboratory for Molecular Sciences, CAS Key Laboratory of Colloid, Interface and Chemical Thermodynamics, CAS Research/Education Center for Excellence in Molecular Sciences, Institute of Chemistry, Chinese Academy of Sciences, Beijing, 100190 P. R. China
School of Chemistry and Chemical Engineering, University of Chinese Academy of Sciences, Beijing, 100049 P. R. China
Search for more papers by this authorDr. Xiaofu Sun
Beijing National Laboratory for Molecular Sciences, CAS Key Laboratory of Colloid, Interface and Chemical Thermodynamics, CAS Research/Education Center for Excellence in Molecular Sciences, Institute of Chemistry, Chinese Academy of Sciences, Beijing, 100190 P. R. China
School of Chemistry and Chemical Engineering, University of Chinese Academy of Sciences, Beijing, 100049 P. R. China
Search for more papers by this authorCorresponding Author
Dr. Qinggong Zhu
Beijing National Laboratory for Molecular Sciences, CAS Key Laboratory of Colloid, Interface and Chemical Thermodynamics, CAS Research/Education Center for Excellence in Molecular Sciences, Institute of Chemistry, Chinese Academy of Sciences, Beijing, 100190 P. R. China
School of Chemistry and Chemical Engineering, University of Chinese Academy of Sciences, Beijing, 100049 P. R. China
Search for more papers by this authorCorresponding Author
Prof. Dr. Buxing Han
Beijing National Laboratory for Molecular Sciences, CAS Key Laboratory of Colloid, Interface and Chemical Thermodynamics, CAS Research/Education Center for Excellence in Molecular Sciences, Institute of Chemistry, Chinese Academy of Sciences, Beijing, 100190 P. R. China
School of Chemistry and Chemical Engineering, University of Chinese Academy of Sciences, Beijing, 100049 P. R. China
Shanghai Key Laboratory of Green Chemistry and Chemical Processes, School of Chemistry and Molecular Engineering, East China Normal University, Shanghai, 200062 P. R. China
Institute of Eco-Chongming, 20 Cuiniao Road, Chenjia Town, Chongming District, Shanghai, 202162 China
Search for more papers by this authorJiahui Bi
Beijing National Laboratory for Molecular Sciences, CAS Key Laboratory of Colloid, Interface and Chemical Thermodynamics, CAS Research/Education Center for Excellence in Molecular Sciences, Institute of Chemistry, Chinese Academy of Sciences, Beijing, 100190 P. R. China
School of Chemistry and Chemical Engineering, University of Chinese Academy of Sciences, Beijing, 100049 P. R. China
Search for more papers by this authorPengsong Li
Beijing National Laboratory for Molecular Sciences, CAS Key Laboratory of Colloid, Interface and Chemical Thermodynamics, CAS Research/Education Center for Excellence in Molecular Sciences, Institute of Chemistry, Chinese Academy of Sciences, Beijing, 100190 P. R. China
School of Chemistry and Chemical Engineering, University of Chinese Academy of Sciences, Beijing, 100049 P. R. China
Search for more papers by this authorJiyuan Liu
Beijing National Laboratory for Molecular Sciences, CAS Key Laboratory of Colloid, Interface and Chemical Thermodynamics, CAS Research/Education Center for Excellence in Molecular Sciences, Institute of Chemistry, Chinese Academy of Sciences, Beijing, 100190 P. R. China
School of Chemistry and Chemical Engineering, University of Chinese Academy of Sciences, Beijing, 100049 P. R. China
Search for more papers by this authorYong Wang
Beijing National Laboratory for Molecular Sciences, CAS Key Laboratory of Colloid, Interface and Chemical Thermodynamics, CAS Research/Education Center for Excellence in Molecular Sciences, Institute of Chemistry, Chinese Academy of Sciences, Beijing, 100190 P. R. China
School of Chemistry and Chemical Engineering, University of Chinese Academy of Sciences, Beijing, 100049 P. R. China
Search for more papers by this authorXinning Song
Beijing National Laboratory for Molecular Sciences, CAS Key Laboratory of Colloid, Interface and Chemical Thermodynamics, CAS Research/Education Center for Excellence in Molecular Sciences, Institute of Chemistry, Chinese Academy of Sciences, Beijing, 100190 P. R. China
School of Chemistry and Chemical Engineering, University of Chinese Academy of Sciences, Beijing, 100049 P. R. China
Search for more papers by this authorDr. Xinchen Kang
Beijing National Laboratory for Molecular Sciences, CAS Key Laboratory of Colloid, Interface and Chemical Thermodynamics, CAS Research/Education Center for Excellence in Molecular Sciences, Institute of Chemistry, Chinese Academy of Sciences, Beijing, 100190 P. R. China
School of Chemistry and Chemical Engineering, University of Chinese Academy of Sciences, Beijing, 100049 P. R. China
Search for more papers by this authorDr. Xiaofu Sun
Beijing National Laboratory for Molecular Sciences, CAS Key Laboratory of Colloid, Interface and Chemical Thermodynamics, CAS Research/Education Center for Excellence in Molecular Sciences, Institute of Chemistry, Chinese Academy of Sciences, Beijing, 100190 P. R. China
School of Chemistry and Chemical Engineering, University of Chinese Academy of Sciences, Beijing, 100049 P. R. China
Search for more papers by this authorCorresponding Author
Dr. Qinggong Zhu
Beijing National Laboratory for Molecular Sciences, CAS Key Laboratory of Colloid, Interface and Chemical Thermodynamics, CAS Research/Education Center for Excellence in Molecular Sciences, Institute of Chemistry, Chinese Academy of Sciences, Beijing, 100190 P. R. China
School of Chemistry and Chemical Engineering, University of Chinese Academy of Sciences, Beijing, 100049 P. R. China
Search for more papers by this authorCorresponding Author
Prof. Dr. Buxing Han
Beijing National Laboratory for Molecular Sciences, CAS Key Laboratory of Colloid, Interface and Chemical Thermodynamics, CAS Research/Education Center for Excellence in Molecular Sciences, Institute of Chemistry, Chinese Academy of Sciences, Beijing, 100190 P. R. China
School of Chemistry and Chemical Engineering, University of Chinese Academy of Sciences, Beijing, 100049 P. R. China
Shanghai Key Laboratory of Green Chemistry and Chemical Processes, School of Chemistry and Molecular Engineering, East China Normal University, Shanghai, 200062 P. R. China
Institute of Eco-Chongming, 20 Cuiniao Road, Chenjia Town, Chongming District, Shanghai, 202162 China
Search for more papers by this authorGraphical Abstract
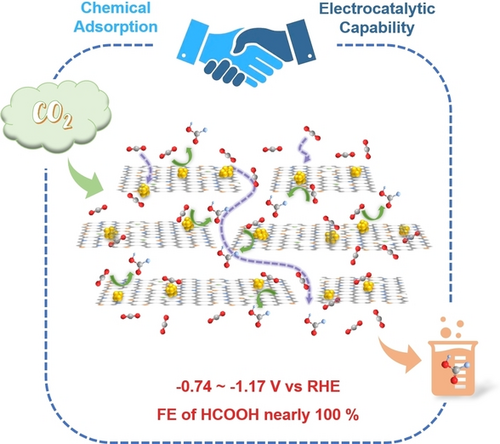
Indium nanoparticles on chitosan-derived N-doped defective graphene (In/N-dG) create bifunctional active centers that integrate chemical adsorption and electrocatalytic capabilities for CO2 electrolysis. The electrocatalyst demonstrated outstanding performance for the CO2 reduction reaction with a nearly 100 % Faradaic efficiency toward HCOOH across a wide potential window and with high current density.
Abstract
Realizing industrial-scale production of HCOOH from the CO2 reduction reaction (CO2RR) is very important, but the current density as well as the electrochemical potential window are still limited to date. Herein, we achieved this by integration of chemical adsorption and electrocatalytic capabilities for the CO2RR via anchoring In nanoparticles (NPs) on biomass-derived substrates to create In/X−C (X=N, P, B) bifunctional active centers. The In NPs/chitosan-derived N-doped defective graphene (In/N-dG) catalyst had outstanding performance for the CO2RR with a nearly 100 % Faradaic efficiency (FE) of HCOOH across a wide potential window. Particularly, at 1.2 A ⋅ cm−2 high current density, the FE of HCOOH was as high as 96.0 %, and the reduction potential was as low as −1.17 V vs RHE. When using a membrane electrode assembly (MEA), a pure HCOOH solution could be obtained at the cathode without further separation and purification. The FE of HCOOH was still up to 93.3 % at 0.52 A ⋅ cm−2, and the HCOOH production rate could reach 9.051 mmol ⋅ h−1 ⋅ cm−2. Our results suggested that the defects and multilayer structure in In/N-dG could not only enhance CO2 chemical adsorption capability, but also trigger the formation of an electron-rich catalytic environment around In sites to promote the generation of HCOOH.
Conflict of interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202307612-sup-0001-misc_information.pdf2.2 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aP. De Luna, C. Hahn, D. Higgins, S. A. Jaffer, T. F. Jaramillo, E. H. Sargent, Science 2019, 364;
- 1bD. U. Nielsen, X.-M. Hu, K. Daasbjerg, T. Skrydstrup, Nat. Catal. 2018, 1, 244–254;
- 1cS. Jia, Q. Zhu, M. Chu, S. Han, R. Feng, J. Zhai, W. Xia, M. He, H. Wu, B. Han, Angew. Chem. Int. Ed. 2021, 60, 10977–10982.
- 2
- 2aK. Sun, K. Yu, J. Fang, Z. Zhuang, X. Tan, Y. Wu, L. Zeng, Z. Zhuang, Y. Pan, C. Chen, Adv. Mater. 2022, 34, 2206478;
- 2bX. Tan, K. Sun, Z. Zhuang, B. Hu, Y. Zhang, Q. Liu, C. He, Z. Xu, C. Chen, H. Xiao, C. Chen, J. Am. Chem. Soc. 2023, 145, 8656–8664;
- 2cW. Guo, X. Tan, J. Bi, L. Xu, D. Yang, C. Chen, Q. Zhu, J. Ma, A. Tayal, J. Ma, Y. Huang, X. Sun, S. Liu, B. Han, J. Am. Chem. Soc. 2021, 143, 6877–6885;
- 2dY. Xie, P. Ou, X. Wang, Z. Xu, Y. C. Li, Z. Wang, J. E. Huang, J. Wicks, C. McCallum, N. Wang, Y. Wang, T. Chen, B. T. W. Lo, D. Sinton, J. C. Yu, Y. Wang, E. H. Sargent, Nat. Catal. 2022, 5, 564–570;
- 2eA. Zhang, Y. Liang, H. Li, X. Zhao, Y. Chen, B. Zhang, W. Zhu, J. Zeng, Nano Lett. 2019, 19, 6547–6553
- 2fX. C. Jiao, Z. X. Hu, L. Li, Y. Wu, K. Zheng, Y. F. Sun, Y. Xie, Sci. China Chem. 2022, 65, 428–440;
- 2gP. Li, J. Bi, J. Liu, Q. Zhu, C. Chen, X. Sun, J. Zhang, B. Han, Nat. Commun. 2022, 13, 1965;
- 2hC. Cao, D. D. Ma, J. F. Gu, X. Xie, G. Zeng, X. Li, S. G. Han, Q. L. Zhu, X. T. Wu, Q. Xu, Angew. Chem. Int. Ed. 2020, 59, 15014–15020.
- 3
- 3aW. Ma, S. Xie, X. G. Zhang, F. Sun, J. Kang, Z. Jiang, Q. Zhang, D. Y. Wu, Y. Wang, Nat. Commun. 2019, 10, 892;
- 3bY. Xing, X. Kong, X. Guo, Y. Liu, Q. Li, Y. Zhang, Y. Sheng, X. Yang, Z. Geng, J. Zeng, Adv. Sci. 2020, 7, 1902989;
- 3cL. Fan, C. Xia, P. Zhu, Y. Lu, H. Wang, Nat. Commun. 2020, 11, 3633.
- 4
- 4aZ. H. Zhu, B. H. Zhao, S. L. Hou, X. L. Jiang, Z. L. Liang, B. Zhang, B. Zhao, Angew. Chem. Int. Ed. 2021, 60, 23394–23402;
- 4bL. P. Chi, Z. Z. Niu, X. L. Zhang, P. P. Yang, J. Liao, F. Y. Gao, Z. Z. Wu, K. B. Tang, M. R. Gao, Nat. Commun. 2021, 12, 5835;
- 4cJ. Zhang, T. Fan, P. Huang, X. Lian, Y. Guo, Z. Chen, X. Yi, Adv. Funct. Mater. 2022, 32, 2113075;
- 4dI. Grigioni, L. K. Sagar, Y. C. Li, G. Lee, Y. Yan, K. Bertens, R. K. Miao, X. Wang, J. Abed, D. H. Won, F. P. García de Arquer, A. H. Ip, D. Sinton, E. H. Sargent, ACS Energy Lett. 2021, 6, 79–84;
- 4eM. Liu, Y. Wang, T. Yu, L. Zhan, X. Zhao, C. Lian, Y. Xiong, X. Xiong, Y. Lei, Sci. Bull. 2023, 68, 1238–1242;
- 4fX. F. Bai, W. Chen, B. Y. Wang, G. H. Wang, W. Wei, Z. Jiao, Y. H. Sun, Acta Phys. Chim. Sin. 2017, 33, 2388–2403.
- 5
- 5aJ. Chang, G. Wang, M. Wang, Q. Wang, B. Li, H. Zhou, Y. Zhu, W. Zhang, M. Omer, N. Orlovskaya, Q. Ma, M. Gu, Z. Feng, G. Wang, Y. Yang, Nat. Energy 2021, 6, 1144–1153;
- 5bQ. Hao, H.-X. Zhong, J.-Z. Wang, K.-H. Liu, J.-M. Yan, Z.-H. Ren, N. Zhou, X. Zhao, H. Zhang, D.-X. Liu, X. Liu, L.-W. Chen, J. Luo, X.-B. Zhang, Nat. Synth. 2022, 1, 719–728;
- 5cA. Wagner, C. D. Sahm, E. Reisner, Nat. Catal. 2020, 3, 775–786.
- 6
- 6aJ. Wang, X. Huang, S. Xi, J. M. Lee, C. Wang, Y. Du, X. Wang, Angew. Chem. Int. Ed. 2019, 58, 13532–13539;
- 6bC. Rogers, W. S. Perkins, G. Veber, T. E. Williams, R. R. Cloke, F. R. Fischer, J. Am. Chem. Soc. 2017, 139, 4052–4061;
- 6cT. Pu, W. Zhang, M. Zhu, Angew. Chem. Int. Ed. 2023, 62, e202212278.
- 7
- 7aQ. Wang, K. Liu, K. Hu, C. Cai, H. Li, H. Li, M. Herran, Y. R. Lu, T. S. Chan, C. Ma, J. Fu, S. Zhang, Y. Liang, E. Cortes, M. Liu, Nat. Commun. 2022, 13, 6082;
- 7bL. Zhang, F. Mao, L. R. Zheng, H. F. Wang, X. H. Yang, H. G. Yang, ACS Catal. 2018, 8, 11035–11041.
- 8Q. Hu, Z. Han, X. Wang, G. Li, Z. Wang, X. Huang, H. Yang, X. Ren, Q. Zhang, J. Liu, C. He, Angew. Chem. Int. Ed. 2020, 59, 19054–19059.
- 9G. Zhang, T. Wang, M. Zhang, L. Li, D. Cheng, S. Zhen, Y. Wang, J. Qin, Z. J. Zhao, J. Gong, Nat. Commun. 2022, 13, 7768.
- 10K. Zhao, X. Nie, H. Wang, S. Chen, X. Quan, H. Yu, W. Choi, G. Zhang, B. Kim, J. G. Chen, Nat. Commun. 2020, 11, 2455.
- 11
- 11aY. Fu, L. Qin, D. Huang, G. Zeng, C. Lai, B. Li, J. He, H. Yi, M. Zhang, M. Cheng, X. Wen, Appl. Catal. B 2019, 255, 117740;
- 11bH. Chen, F. Dong, S. D. Minteer, Nat. Catal. 2020, 3, 225–244.
- 12
- 12aD. E. F. Oliveira, J. A. O. Chagas, A. L. de Lima, C. J. A. Mota, Ind. Eng. Chem. Res. 2022, 61, 10522–10530;
- 12bM. Borjian Boroujeni, M. S. Laeini, M. T. Nazeri, A. Shaabani, Catal. Lett. 2019, 149, 2089–2097;
- 12cS. M. Rafigh, A. Heydarinasab, ACS Sustainable Chem. Eng. 2017, 5, 10379–10386.
- 13
- 13aA. Primo, F. Neatu, M. Florea, V. Parvulescu, H. Garcia, Nat. Commun. 2014, 5, 5291;
- 13bP. Mohammadi, M. Heravi, M. Daraie, Sci. Rep. 2021, 11, 17124.
- 14M. Zhu, C. Cao, J. Chen, Y. Sun, R. Ye, J. Xu, Y.-F. Han, ACS Appl. Energ. Mater. 2019, 2, 2435–2440.
- 15
- 15aH. Yang, Y. Wu, G. Li, Q. Lin, Q. Hu, Q. Zhang, J. Liu, C. He, J. Am. Chem. Soc. 2019, 141, 12717–12723;
- 15bH. Zhang, J. Li, S. Xi, Y. Du, X. Hai, J. Wang, H. Xu, G. Wu, J. Zhang, J. Lu, J. Wang, Angew. Chem. Int. Ed. 2019, 58, 14871–14876;
- 15cP. Lu, X. Tan, H. Zhao, Q. Xiang, K. Liu, X. Zhao, X. Yin, X. Li, X. Hai, S. Xi, A. T. S. Wee, S. J. Pennycook, X. Yu, M. Yuan, J. Wu, G. Zhang, S. C. Smith, Z. Yin, ACS Nano 2021, 15, 5671–5678.
- 16
- 16aK. M. Cho, K. H. Kim, K. Park, C. Kim, S. Kim, A. Al-Saggaf, I. Gereige, H.-T. Jung, ACS Catal. 2017, 7, 7064–7069;
- 16bJ. Leverett, R. Daiyan, L. Gong, K. Iputera, Z. Tong, J. Qu, Z. Ma, Q. Zhang, S. Cheong, J. Cairney, R. S. Liu, X. Lu, Z. Xia, L. Dai, R. Amal, ACS Nano 2021, 15, 12006–12018.
- 17J. Yang, D. Guo, S. Zhao, Y. Lin, R. Yang, D. Xu, N. Shi, X. Zhang, L. Lu, Y. Q. Lan, J. Bao, M. Han, Small 2019, 15, 1804546.
- 18
- 18aT. V. Tam, S. G. Kang, M. H. Kim, S. G. Lee, S. H. Hur, J. S. Chung, W. M. Choi, Adv. Energy Mater. 2019, 1900945;
- 18bS. Suresh Balaji, S. Mohammad Tauquir, M. Karnan, M. Moorthy, M. Sathish, ChemistrySelect 2020, 5, 9825–9833.
- 19
- 19aR. Kou, Y. Shao, D. Mei, Z. Nie, D. Wang, C. Wang, V. V. Viswanathan, S. Park, I. A. Aksay, Y. Lin, Y. Wang, J. Liu, J. Am. Chem. Soc. 2011, 133, 2541–2547;
- 19bY. Zhang, K. Li, M. Chen, J. Wang, J. Liu, Y. Zhang, ACS Appl. Nano Mater. 2020, 3, 257–263.
- 20
- 20aS. Yan, C. Peng, C. Yang, Y. Chen, J. Zhang, A. Guan, X. Lv, H. Wang, Z. Wang, T. K. Sham, Q. Han, G. Zheng, Angew. Chem. Int. Ed. 2021, 60, 25741–25745;
- 20bT. Zheng, C. Liu, C. Guo, M. Zhang, X. Li, Q. Jiang, W. Xue, H. Li, A. Li, C. W. Pao, J. Xiao, C. Xia, J. Zeng, Nat. Nanotechnol. 2021, 16, 1386–1393;
- 20cL. Lin, X. He, X. G. Zhang, W. Ma, B. Zhang, D. Wei, S. Xie, Q. Zhang, X. Yi, Y. Wang, Angew. Chem. Int. Ed. 2023, 62, e202214959.