[2+2+1+1] Cycloaddition for de novo Synthesis of Densely Functionalized Phenols
Dr. Wei Wei
Department of Chemistry, The Hong Kong University of Science and Technology, Clear Water Bay, Kowloon, Hong Kong, China
These authors contributed equally to this work.
Search for more papers by this authorKa Key Cheung
Department of Chemistry, The Hong Kong University of Science and Technology, Clear Water Bay, Kowloon, Hong Kong, China
These authors contributed equally to this work.
Search for more papers by this authorDr. Ran Lin
Department of Chemistry, The Hong Kong University of Science and Technology, Clear Water Bay, Kowloon, Hong Kong, China
Key Laboratory of Biopesticide and Chemical Biology (Ministry of Education), College of Plant Protection, Fujian Agriculture and Forestry University, Fuzhou, Fujian, 350002 China
Search for more papers by this authorLam Cheung Kong
Department of Chemistry, The Hong Kong University of Science and Technology, Clear Water Bay, Kowloon, Hong Kong, China
Search for more papers by this authorKa Lok Chan
Department of Chemistry, The Hong Kong University of Science and Technology, Clear Water Bay, Kowloon, Hong Kong, China
Search for more papers by this authorDr. Herman H. Y. Sung
Department of Chemistry, The Hong Kong University of Science and Technology, Clear Water Bay, Kowloon, Hong Kong, China
Search for more papers by this authorCorresponding Author
Prof. Dr. Ian D. Williams
Department of Chemistry, The Hong Kong University of Science and Technology, Clear Water Bay, Kowloon, Hong Kong, China
Search for more papers by this authorCorresponding Author
Prof. Dr. Rongbiao Tong
Department of Chemistry, The Hong Kong University of Science and Technology, Clear Water Bay, Kowloon, Hong Kong, China
Search for more papers by this authorCorresponding Author
Prof. Dr. Zhenyang Lin
Department of Chemistry, The Hong Kong University of Science and Technology, Clear Water Bay, Kowloon, Hong Kong, China
Search for more papers by this authorCorresponding Author
Prof. Dr. Guochen Jia
Department of Chemistry, The Hong Kong University of Science and Technology, Clear Water Bay, Kowloon, Hong Kong, China
Search for more papers by this authorDr. Wei Wei
Department of Chemistry, The Hong Kong University of Science and Technology, Clear Water Bay, Kowloon, Hong Kong, China
These authors contributed equally to this work.
Search for more papers by this authorKa Key Cheung
Department of Chemistry, The Hong Kong University of Science and Technology, Clear Water Bay, Kowloon, Hong Kong, China
These authors contributed equally to this work.
Search for more papers by this authorDr. Ran Lin
Department of Chemistry, The Hong Kong University of Science and Technology, Clear Water Bay, Kowloon, Hong Kong, China
Key Laboratory of Biopesticide and Chemical Biology (Ministry of Education), College of Plant Protection, Fujian Agriculture and Forestry University, Fuzhou, Fujian, 350002 China
Search for more papers by this authorLam Cheung Kong
Department of Chemistry, The Hong Kong University of Science and Technology, Clear Water Bay, Kowloon, Hong Kong, China
Search for more papers by this authorKa Lok Chan
Department of Chemistry, The Hong Kong University of Science and Technology, Clear Water Bay, Kowloon, Hong Kong, China
Search for more papers by this authorDr. Herman H. Y. Sung
Department of Chemistry, The Hong Kong University of Science and Technology, Clear Water Bay, Kowloon, Hong Kong, China
Search for more papers by this authorCorresponding Author
Prof. Dr. Ian D. Williams
Department of Chemistry, The Hong Kong University of Science and Technology, Clear Water Bay, Kowloon, Hong Kong, China
Search for more papers by this authorCorresponding Author
Prof. Dr. Rongbiao Tong
Department of Chemistry, The Hong Kong University of Science and Technology, Clear Water Bay, Kowloon, Hong Kong, China
Search for more papers by this authorCorresponding Author
Prof. Dr. Zhenyang Lin
Department of Chemistry, The Hong Kong University of Science and Technology, Clear Water Bay, Kowloon, Hong Kong, China
Search for more papers by this authorCorresponding Author
Prof. Dr. Guochen Jia
Department of Chemistry, The Hong Kong University of Science and Technology, Clear Water Bay, Kowloon, Hong Kong, China
Search for more papers by this authorGraphical Abstract
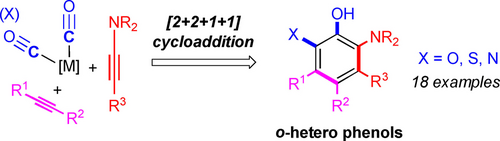
Densely functionalized phenols can be easily obtained through formal [2+2+1+1] cycloaddition of two different alkynes and two molecules of CO. The new benzannulation strategy allows efficient regioselective installation of up to five different substituents on a phenol ring. The resulting phenols have a substitution pattern different from and complementary to those obtained from Dötz and Danheiser benzannulations.
Abstract
A unique benzannulation strategy for regioselective de novo synthesis of densely functionalized phenols is described. Through metal-mediated formal [2+2+1+1] cycloaddition of two different alkynes and two molecules of CO, a series of densely functionalized phenols were obtained. The benzannulation strategy allows efficient regioselective installation up to five different substituents on a phenol ring. The resulting phenols have a substitution pattern different from those obtained from Dötz and Danheiser benzannulations.
Conflict of interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
Research data are not shared.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For reviews, see:
- 1aS. Quideau, D. Deffieux, C. Douat-Casassus, L. Pouységu, Angew. Chem. Int. Ed. 2011, 50, 586–621;
- 1bP. M. Dewick, Medicinal natural products: a biosynthetic approach, 3rd ed, John Wiley & Sons, Ltd, Chichester, UK, 2009;
10.1002/9780470742761 Google Scholar
- 1cZ. Rappoport, The chemistry of phenols, John Wiley & Sons, Ltd, Chichester, UK, 2003.
10.1002/0470857277 Google Scholar
- 2Selected examples:
- 2aT. A. Hong Nguyen, D.-R. Hou, Org. Lett. 2021, 23, 8127–8131;
- 2bX. Zhang, T. T. Wang, Q. L. Xu, Y. Xiong, L. Zhang, H. Han, K. Xu, W. J. Guo, Q. Xu, R. X. Tan, H. M. Ge, Angew. Chem. Int. Ed. 2018, 57, 8184–8188;
- 2cS. Xu, B. Nijampatnam, S. Dutta, S. E. Velu, Mar. Drugs 2016, 14, 17;
- 2dJ. Kretz, D. Kerwat, V. Schubert, S. Grätz, A. Pesic, S. Semsary, S. Cociancich, M. Royer, R. D. Süssmuth, Angew. Chem. Int. Ed. 2015, 54, 1969–1973;
- 2eK. Gebauer, A. Fürstner, Angew. Chem. Int. Ed. 2014, 53, 6393–6396.
- 3Y. Satkar, L. F. I–Ledesma, N. Mali, D. Patil, P. Navarro-Santos, L. A. Segura-Quezada, P. I. Ramírez-Morales, C. R. Solorio-Alvarado, J. Org. Chem. 2019, 84, 4149–4164.
- 4
- 4aY. Ishida, I. Nakamura, M. Terada, J. Am. Chem. Soc. 2018, 140, 8629–8633;
- 4bY. Wu, Z. Chen, Y. Yang, W. Zhu, B. Zhou, J. Am. Chem. Soc. 2018, 140, 42–45.
- 5
- 5aL.-C. Xu, K.-M. Liu, X.-F. Duan, Adv. Synth. Catal. 2019, 361, 5421–5427;
- 5bP. Camargo Solórzano, F. Brigante, A. B. Pierini, L. B. Jimenez, J. Org. Chem. 2018, 83, 7867–7877;
- 5cM. Y. Zhang, R. A. Barrow, J. Org. Chem. 2018, 83, 6776–6782.
- 6
- 6aH. Chen, M. Yang, G. Wang, L. Gao, Z. Ni, J. Zou, S. Li, Org. Lett. 2021, 23, 5533–5538;
- 6bZ. Huang, J.-P. Lumb, ACS Catal. 2019, 9, 521–555;
- 6cC. K. Rank, B. Özkaya, F. W. Patureau, Org. Lett. 2019, 21, 6830–6834;
- 6dH. Lee, M. V. Mane, H. Ryu, D. Sahu, M.-H. Baik, C. S. Yi, J. Am. Chem. Soc. 2018, 140, 10289–10296;
- 6eP. Xiao, C.-X. Li, W.-H. Fang, G. Cui, W. Thiel, J. Am. Chem. Soc. 2018, 140, 15099–15113;
- 6fC. Yu, F. W. Patureau, Angew. Chem. Int. Ed. 2018, 57, 11807–11811;
- 6gB. Chattopadhyay, J. E. Dannatt, I. L. Andujar-De Sanctis, K. A. Gore, R. E. Maleczka, D. A. Singleton, M. R. Smith, J. Am. Chem. Soc. 2017, 139, 7864–7871;
- 6hJ.-L. Dai, N.-Q. Shao, J. Zhang, R.-P. Jia, D.-H. Wang, J. Am. Chem. Soc. 2017, 139, 12390–12393;
- 6iA. Sagadevan, V. P. Charpe, A. Ragupathi, K. C. Hwang, J. Am. Chem. Soc. 2017, 139, 2896–2899;
- 6jY. Hua, P. Asgari, T. Avullala, J. Jeon, J. Am. Chem. Soc. 2016, 138, 7982–7991;
- 6kP. Wang, M. E. Farmer, X. Huo, P. Jain, P.-X. Shen, M. Ishoey, J. E. Bradner, S. R. Wisniewski, M. D. Eastgate, J.-Q. Yu, J. Am. Chem. Soc. 2016, 138, 9269–9276.
- 7
- 7aJ. Börgel, L. Tanwar, F. Berger, T. Ritter, J. Am. Chem. Soc. 2018, 140, 16026–16031;
- 7bN. Viswanadh, G. S. Ghotekar, M. B. Thoke, R. Velayudham, A. C. Shaikh, M. Karthikeyan, M. Muthukrishnan, Chem. Commun. 2018, 54, 2252–2255;
- 7cL. Yang, Z. Huang, G. Li, W. Zhang, R. Cao, C. Wang, J. Xiao, D. Xue, Angew. Chem. Int. Ed. 2018, 57, 1968–1972;
- 7dP. S. Fier, K. M. Maloney, Angew. Chem. Int. Ed. 2017, 56, 4478–4482;
- 7eY. Wu, B. Zhou, Org. Lett. 2017, 19, 3532–3535;
- 7fA. Maji, B. Bhaskararao, S. Singha, R. B. Sunoj, D. Maiti, Chem. Sci. 2016, 7, 3147–3153;
- 7gS. Xia, L. Gan, K. Wang, Z. Li, D. Ma, J. Am. Chem. Soc. 2016, 138, 13493–13496;
- 7hF. Yang, K. Rauch, K. Kettelhoit, L. Ackermann, Angew. Chem. Int. Ed. 2014, 53, 11285–11288.
- 8For reviews, see:
- 8aJ. A. M. Vargas, D. P. Day, A. C. B. Burtoloso, Eur. J. Org. Chem. 2021, 741–756;
- 8bT. N. Poudel, R. J. I. Tamargo, H. Cai, Y. R. Lee, Asian J. Org. Chem. 2018, 7, 985–1005.
- 9K. H. Dötz, Angew. Chem. Int. Ed. Engl. 1975, 14, 644–645. For recently reviews, see:
- 9aR. A. Fernandes, A. Kumari, R. S. Pathare, Synlett 2020, 31, 403–420;
- 9bJ. Barluenga, E. Aguilar, in Advances in Organometallic Chemistry, Vol. 67 (Ed.: P. J. Pérez), Academic Press, 2017, pp. 1–150;
- 9cL. Chupak, in Name Reactions for Carbocyclic Ring Formations (Ed.: J. J. Li), John Wiley & Sons, Inc, New Jersey, 2010, pp. 309–323;
- 9dM. L. Waters, W. D. Wulff, in Organic Reactions, Vol. 70, John Wiley & Sons, Inc, 2008, pp. 121–623.
- 10Examples of recent work:
- 10aN. Majumdar, K. A. Korthals, W. D. Wulff, J. Am. Chem. Soc. 2012, 134, 1357–1362;
- 10bK. A. Korthals, W. D. Wulff, J. Am. Chem. Soc. 2008, 130, 2898–2899.
- 11
- 11aP.-h. Chen, G. Dong, Chem. Eur. J. 2016, 22, 18290–18315;
- 11bR. L. Danheiser, in Category 3, Compounds with Four and Three Carbon Heteroatom Bonds, Vol. 23, 1st ed, Georg Thieme Verlag KG, Stuttgart, 2006.
- 12
- 12aT. Y. Lam, Y.-P. Wang, R. L. Danheiser, J. Org. Chem. 2013, 78, 9396–9414;
- 12bX. Y. Mak, A. L. Crombie, R. L. Danheiser, J. Org. Chem. 2011, 76, 1852–1873.
- 13
- 13aC. C. Forneris, Y.-P. Wang, G. Mamaliga, T. P. Willumstad, R. L. Danheiser, Org. Lett. 2018, 20, 6318–6322;
- 13bT. P. Willumstad, P. D. Boudreau, R. L. Danheiser, J. Org. Chem. 2015, 80, 11794–11805;
- 13cT. P. Willumstad, O. Haze, X. Y. Mak, T. Y. Lam, Y.-P. Wang, R. L. Danheiser, J. Org. Chem. 2013, 78, 11450–11469;
- 13dR. L. Danheiser, R. G. Brisbois, J. J. Kowalczyk, R. F. Miller, J. Am. Chem. Soc. 1990, 112, 3093–3100.
- 14W. F. Austin, Y. Zhang, R. L. Danheiser, Org. Lett. 2005, 7, 3905–3908.
- 15See, for example:
- 15aE. Peña-Cabrera, L. S. Liebeskind, J. Org. Chem. 2002, 67, 1689–1691;
- 15bR. Tiedemann, P. Turnbull, H. W. Moore, J. Org. Chem. 1999, 64, 4030–4041;
- 15cD. J. Krysan, A. Gurski, L. S. Liebeskind, J. Am. Chem. Soc. 1992, 114, 1412–1418.
- 16S. Serra, C. Fuganti, E. Brenna, Chem. Eur. J. 2007, 13, 6782–6791.
- 17T. Kuzuguchi, Y. Yabuuchi, T. Yoshimura, J.-i. Matsuo, Org. Biomol. Chem. 2017, 15, 5268–5271.
- 18
- 18aM. G. Weaver, W.-J. Bai, S. K. Jackson, T. R. Pettus, Org. Lett. 2014, 16, 1294–1297;
- 18bS. Danishefsky, Acc. Chem. Res. 1981, 14, 400–406.
- 19V. Gevorgyan, L. G. Quan, Y. Yamamoto, J. Org. Chem. 1998, 63, 1244–1247.
- 20W. Song, S. A. Blaszczyk, J. Liu, S. Wang, W. Tang, Org. Biomol. Chem. 2017, 15, 7490–7504.
- 21
- 21aS. A. Blaszczyk, D. A. Glazier, W. Tang, Acc. Chem. Res. 2020, 53, 231–243;
- 21bL.-J. Wu, L.-F. Yang, J.-H. Li, Q.-A. Wang, Chem. Commun. 2020, 56, 1669–1672;
- 21cL.-J. Wu, R.-J. Song, S. Luo, J.-H. Li, Angew. Chem. Int. Ed. 2018, 57, 13308–13312.
- 22
- 22aP. A. Evans, A. J. Burnie, D. E. Negru, Org. Lett. 2014, 16, 4356–4359;
- 22bS. Kim, Y. K. Chung, Org. Lett. 2014, 16, 4352–4355;
- 22cG.-Q. Chen, M. Shi, Chem. Commun. 2013, 49, 698–700;
- 22dW. Zhao, J. Zhang, Org. Lett. 2011, 13, 688–691.
- 23
- 23aP. A. Wender, G. G. Gamber, R. D. Hubbard, S. M. Pham, L. Zhang, J. Am. Chem. Soc. 2005, 127, 2836–2837;
- 23bA. Padwa, S. L. Xu, J. Am. Chem. Soc. 1992, 114, 5881–5882;
- 23cS. H. Cho, L. S. Liebeskind, J. Org. Chem. 1987, 52, 2631–2634.
- 24M.-G. Rong, T.-Z. Qin, X.-R. Liu, H.-F. Wang, W. Zi, Org. Lett. 2018, 20, 6289–6293.
- 25
- 25aC.-B. Miao, M.-L. Chen, X.-Q. Sun, H.-T. Yang, Synthesis 2019, 51, 2945–2958;
- 25bZ. Hu, J. Dong, Y. Men, Y. Li, X. Xu, Chem. Commun. 2017, 53, 1739–1742;
- 25cT. N. Poudel, Y. R. Lee, Chem. Sci. 2015, 6, 7028–7033.
- 26
- 26aH. Yu, Z. Zhang, X. Zhang, Y. Xu, D. Huo, L. Zhang, W. Wang, J. Org. Chem. 2022, 87, 2985–2996;
- 26bL. Pan, Q. Liu, Synlett 2011, 2011, 1073–1080;
- 26cX. Bi, D. Dong, Q. Liu, W. Pan, L. Zhao, B. Li, J. Am. Chem. Soc. 2005, 127, 4578–4579.
- 27
- 27aT. Stalling, W. R. R. Harker, A.-L. Auvinet, E. J. Cornel, J. P. A. Harrity, Chem. Eur. J. 2015, 21, 2701–2704;
- 27bA.-L. Auvinet, J. P. A. Harrity, Angew. Chem. Int. Ed. 2011, 50, 2769–2772;
- 27cM. A. Huffman, L. S. Liebeskind, J. Am. Chem. Soc. 1991, 113, 2771–2772;
- 27dM. A. Huffman, L. S. Liebeskind, J. Am. Chem. Soc. 1990, 112, 8617–8618.
- 28
- 28aA. S. K. Hashmi, T. Häffner, M. Rudolph, F. Rominger, Chem. Eur. J. 2011, 17, 8195–8201;
- 28bY. Chen, W. Yan, N. G. Akhmedov, X. Shi, Org. Lett. 2010, 12, 344–347;
- 28cA. S. K. Hashmi, J. P. Weyrauch, E. Kurpejović, T. M. Frost, B. Miehlich, W. Frey, J. W. Bats, Chem. Eur. J. 2006, 12, 5806–5814.
- 29
- 29aW. T. Teo, W. Rao, C. J. H. Ng, S. W. Y. Koh, P. W. H. Chan, Org. Lett. 2014, 16, 1248–1251;
- 29bC. Li, Y. Zeng, H. Zhang, J. Feng, Y. Zhang, J. Wang, Angew. Chem. Int. Ed. 2010, 49, 6413–6417.
- 30For other reactions of metallacyclobutadienes, see, for example;
- 30aA. Haack, J. Hillenbrand, M. Leutzsch, M. van Gastel, F. Neese, A. Fürstner, J. Am. Chem. Soc. 2021, 143, 5643–5648;
- 30bC. Zhu, Y. Yang, M. Luo, C. Yang, J. Wu, L. Chen, G. Liu, T. Wen, J. Zhu, H. Xia, Angew. Chem. Int. Ed. 2015, 54, 6181–6185;
- 30cG. R. Clark, G.-L. Lu, W. R. Roper, L. J. Wright, Organometallics 2007, 26, 2167–2177.
- 31W. Wei, X. Xu, H. H. Y. Sung, I. D. Williams, Z. Lin, G. Jia, Angew. Chem. Int. Ed. 2022, 61, e202202886.
- 32Deposition numbers 2261564 (complex 4 e), 2261565 (complex 4 j), 2261566 (complex 4 k), 2264116 (complex 4 k’), 2261567 (complex 6 a), 2261577 (complex 6 d), 2261573 (complex 6 f), 2261571 (complex 6 g), 2261569 (complex 6 o), 2261574 (complex 6 p), 2261570 (complex 6 q), 2261575 (complex 6 r) and 2261579 (compound 7 p) contain the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service.
- 33
- 33aR. Lin, K. H. Lee, K. C. Poon, H. H. Sung, I. D. Williams, Z. Lin, G. Jia, Chem. Eur. J. 2014, 20, 14885–14899;
- 33bK. C. Poon, L. Liu, T. Guo, J. Li, H. H. Sung, I. D. Williams, Z. Lin, G. Jia, Angew. Chem. Int. Ed. 2010, 122, 2819–2822.
- 34See, for examles,
- 34aR. Aumann, H. Heinen, R. Goddard, C. Krüger, Chem. Ber. 1991, 124, 2587–2593;
- 34bR. Aumann, H. Heinen, P. Hinterding, N. Sträter, B. Krebs, Chem. Ber. 1991, 124, 1229–1236;
- 34cK.-H. Dötz, D. Neugebauer, Angew. Chem. Int. Ed. Engl. 1978, 17, 851–852;
- 34dK. H. Dötz, I. Pruskil, Chem. Ber. 1978, 111, 2059–2063;
- 34eK. H. Dötz, Chem. Ber. 1977, 110, 78–85;
- 34fK. H. Dötz, I. Pruskil, J. Organomet. Chem. 1977, 132, 115–120;
- 34gK. H. Dötz, C. G. Kreiter, Chem. Ber. 1976, 109, 2026–2032.
- 35The low yield for 4 e is caused by the poor nucleophilicity of the p-Cresol. For similar observations, see, V. Plantevin, A. Wojcicki, J. Organomet. Chem. 2004, 689, 2000–2012.
- 36R. Lin, K.-H. Lee, H. H. Sung, I. D. Williams, Z. Lin, G. Jia, Organometallics 2015, 34, 167–176.
- 37Reactions of thiocarbenes with aminoalkyns usually give similar insertion product;
- 37aK. Heinz Dötz, V. Leue, J. Organomet. Chem. 1991, 407, 337–351;
- 37bR. Aumann, J. Schröder, H. Heinen, Chem. Ber. 1990, 123, 1369–1374;
- 37cA. Yamashita, A. Toy, N. B. Ghazal, C. R. Muchmore, J. Org. Chem. 1989, 54, 4481–4483.
- 38Reactions of aminocarbenes with aminoalkyns usually give similar insertion product or indene derivations. R. Aumann, H. Heinen, C. Krüger, P. Betz, Chem. Ber. 1990, 123, 605–610.