Defluorinative Multi-Functionalization of Fluoroaryl Sulfoxides Enabled by Fluorine-Assisted Temporary Dearomatization
Mengjie Hu
Key Laboratory of the Ministry of Education for Advanced Catalysis Materials, Zhejiang Normal University, Jinhua, 321004 China
State Key Laboratory of Fine Chemicals, Dalian University of Technology, Dalian, 116023 China
These authors contributed equally to this work.
Search for more papers by this authorYuchen Liang
Key Laboratory of the Ministry of Education for Advanced Catalysis Materials, Zhejiang Normal University, Jinhua, 321004 China
These authors contributed equally to this work.
Search for more papers by this authorLiying Ru
Key Laboratory of the Ministry of Education for Advanced Catalysis Materials, Zhejiang Normal University, Jinhua, 321004 China
These authors contributed equally to this work.
Search for more papers by this authorSheng Ye
Key Laboratory of the Ministry of Education for Advanced Catalysis Materials, Zhejiang Normal University, Jinhua, 321004 China
Search for more papers by this authorLei Zhang
Key Laboratory of the Ministry of Education for Advanced Catalysis Materials, Zhejiang Normal University, Jinhua, 321004 China
Search for more papers by this authorXin Huang
Key Laboratory of the Ministry of Education for Advanced Catalysis Materials, Zhejiang Normal University, Jinhua, 321004 China
Search for more papers by this authorProf. Ming Bao
State Key Laboratory of Fine Chemicals, Dalian University of Technology, Dalian, 116023 China
Search for more papers by this authorLichun Kong
Key Laboratory of the Ministry of Education for Advanced Catalysis Materials, Zhejiang Normal University, Jinhua, 321004 China
Search for more papers by this authorCorresponding Author
Prof. Bo Peng
Key Laboratory of the Ministry of Education for Advanced Catalysis Materials, Zhejiang Normal University, Jinhua, 321004 China
Key Laboratory of Chemical Biology & Traditional Chinese Medicine Research (Ministry of Education), Hunan Normal University, Changsha, 410081 China
Search for more papers by this authorMengjie Hu
Key Laboratory of the Ministry of Education for Advanced Catalysis Materials, Zhejiang Normal University, Jinhua, 321004 China
State Key Laboratory of Fine Chemicals, Dalian University of Technology, Dalian, 116023 China
These authors contributed equally to this work.
Search for more papers by this authorYuchen Liang
Key Laboratory of the Ministry of Education for Advanced Catalysis Materials, Zhejiang Normal University, Jinhua, 321004 China
These authors contributed equally to this work.
Search for more papers by this authorLiying Ru
Key Laboratory of the Ministry of Education for Advanced Catalysis Materials, Zhejiang Normal University, Jinhua, 321004 China
These authors contributed equally to this work.
Search for more papers by this authorSheng Ye
Key Laboratory of the Ministry of Education for Advanced Catalysis Materials, Zhejiang Normal University, Jinhua, 321004 China
Search for more papers by this authorLei Zhang
Key Laboratory of the Ministry of Education for Advanced Catalysis Materials, Zhejiang Normal University, Jinhua, 321004 China
Search for more papers by this authorXin Huang
Key Laboratory of the Ministry of Education for Advanced Catalysis Materials, Zhejiang Normal University, Jinhua, 321004 China
Search for more papers by this authorProf. Ming Bao
State Key Laboratory of Fine Chemicals, Dalian University of Technology, Dalian, 116023 China
Search for more papers by this authorLichun Kong
Key Laboratory of the Ministry of Education for Advanced Catalysis Materials, Zhejiang Normal University, Jinhua, 321004 China
Search for more papers by this authorCorresponding Author
Prof. Bo Peng
Key Laboratory of the Ministry of Education for Advanced Catalysis Materials, Zhejiang Normal University, Jinhua, 321004 China
Key Laboratory of Chemical Biology & Traditional Chinese Medicine Research (Ministry of Education), Hunan Normal University, Changsha, 410081 China
Search for more papers by this authorGraphical Abstract
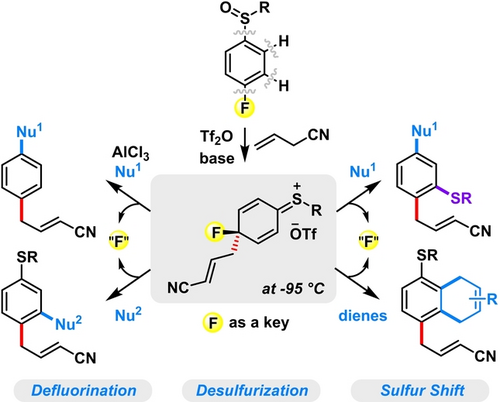
The potential reactivities of fluoroaryl sulfoxides have been unlocked by precisely constructing and elaborating their temporarily dearomatized intermediates via a robust [5,5]-sulfonium-rearrangement-triggered dearomatization/rearomatization process. Impressively, fluorine has shown its irreplaceable role for the success of the reaction.
Abstract
Owing to its unique physical properties, fluorine is often used to open up new reaction channels. In this report, we establish a cooperation of [5,5]-rearrangement and fluorine-assisted temporary dearomatization for arene multi-functionalization. Specifically, the [5,5]-rearrangement of fluoroaryl sulfoxides with β,γ-unsaturated nitriles generates an intriguing dearomatized sulfonium species which is short-lived but exhibits unusually high electrophilicity and thus can be instantly trapped by nucleophiles and dienes at a remarkably low temperature (−95 °C) to produce four types of valuable multi-functionalized benzenes, respectively, involving appealing processes of defluorination, desulfurization, and sulfur shift. Mechanistic studies indicate that the use of fluorine on arenes not only circumvents the generally inevitable [3,3]-rearrangement but also impedes the undesired rearomatization process, thus provides a precious space for constructing and elaborating the temporarily dearomatized fluorinated sulfonium species.
Conflict of interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202306914-sup-0001-misc_information.pdf16.5 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aModern Fluoroorganic Chemistry: Synthesis, Reactivity, Applications (Ed.: P. Kirsch), Wiley-VCH, Weinheim, 2013;
- 1bOrganometallic Fluorine Chemistry (Eds.: T. Braun, R. P. Hughes), Springer, Cham, 2015;
- 1cD. O'Hagan, Chem. Soc. Rev. 2008, 37, 308.
- 2J. Dong, L. Krasnova, M. G. Finn, K. B. Sharpless, Angew. Chem. Int. Ed. 2014, 53, 9430.
- 3W. Zhang, C. Ni, J. Hu, Selective Fluoroalkylation of Organic Compounds by Tackling the “Negative Fluorine Effect”. In Fluorous Chemistry, Vol. 308 (Eds.: I. T. Horváth), Springer, Heidelberg, 2012, pp 25–44.
- 4Y. Zhao, B. Gao, J. Hu, J. Am. Chem. Soc. 2012, 134, 5790.
- 5
- 5aX. Huang, Y. Zhang, C. Zhang, L. Zhang, Y. Xu, L. Kong, Z.-X. Wang, B. Peng, Angew. Chem. Int. Ed. 2019, 58, 5956;
- 5bX. Huang, Y. Zhang, W. Liang, Q. Zhang, Y. Zhan, L. Kong, B. Peng, Chem. Sci. 2020, 11, 3048.
- 6
- 6aN. Zhang, Y. Ye, L. Bai, J. Liu, H. Wang, X. Luan, Chin. Chem. Lett. 2022, 33, 2411;
- 6bV. N. Kovtonyuk, L. S. Kobrina, J. Fluorine Chem. 1994, 66, 219;
- 6cV. N. Kovtonyuk, L. S. Kobrina, J. Fluorine Chem. 1993, 63, 243;
- 6dV. N. Kovtonyuk, L. S. Kobrina, G. G. Yakobson, J. Fluorine Chem. 1985, 28, 89.
- 7
- 7aH. Egami, T. Rouno, T. Niwa, K. Masuda, K. Yamashita, Y. Hamashima, Angew. Chem. Int. Ed. 2020, 59, 14101;
- 7bR. J. Phipps, F. D. Toste, J. Am. Chem. Soc. 2013, 135, 1268;
- 7cP. Pahari, B. Senapati, D. Mal, Tetrahedron Lett. 2007, 48, 2635.
- 8A. Bondi, J. Phys. Chem. 1964, 68, 441.
- 9D. Bergmann, J. Hinze, Struct. Bonding 1987, 66, 145.
- 10
- 10aG. M. Brooke, J. A. K. J. Ferguson, J. Chem. Soc. Perkin Trans. 1 1987, 2091;
- 10bG. M. Brooke, J. A. K. J. Ferguson, J. Chem. Soc. Perkin Trans. 1 1988, 2023.
- 11
- 11aK. Okamoto, K. Nogi, H. Yorimitsu, Org. Lett. 2020, 22, 5540;
- 11bK. Okamoto, M. Hori, T. Yanagi, K. Murakami, K. Nogi, H. Yorimitsu, Angew. Chem. Int. Ed. 2018, 57, 14230;
- 11cK. Okamoto, K. Nogi, J. Shimokawa, H. Yorimitsu, Chem. Eur. J. 2020, 26, 5615;
- 11dT. Yanagi, H. Yorimitsu, J. Synth Org, Chem. Jpn. 2021, 79, 427.
- 12
- 12aY. Liang, B. Peng, Acc. Chem. Res. 2022, 55, 2103;
- 12bL. Shang, Y. Chang, F. Luo, J.-N. He, X. Huang, L. Zhang, L. Kong, K. Li, B. Peng, J. Am. Chem. Soc. 2017, 139, 4211;
- 12cL. Zhang, J.-N. He, Y. Liang, M. Hu, L. Shang, X. Huang, L. Kong, Z.-X. Wang, B. Peng, Angew. Chem. Int. Ed. 2019, 58, 5316;
- 12dM. Chen, Y. Liang, T. Dong, W. Liang, Y. Liu, Y. Zhang, X. Huang, L. Kong, Z.-X. Wang, B. Peng, Angew. Chem. Int. Ed. 2021, 60, 2339.
- 13
- 13aT. Yanagi, K. Nogi, H. Yorimitsu, Tetrahedron Lett. 2018, 59, 2951;
- 13bH. Yorimitsu, Chem. Rec. 2017, 17, 1156;
- 13cA. P. Pulis, D. J. Procter, Angew. Chem. Int. Ed. 2016, 55, 9842;
- 13dD. Kaiser, I. Klose, R. Oost, J. Neuhaus, N. Maulide, Chem. Rev. 2019, 119, 8701;
- 13eL. Zhang, M. Hu, B. Peng, Synlett 2019, 30, 2203;
- 13fZ. He, A. P. Pulis, G. J. P. Perry, D. J. Procter, Phosphorus Sulfur Silicon Relat. Elem. 2019, 194, 669;
- 13gG. J. P. Perry, H. Yorimitsu, ACS Sustainable Chem. Eng. 2022, 10, 2569;
- 13hH. Yorimitsu, G. J. Perry, Proc. Jpn. Acad. Ser. B 2022, 98, 190;
- 13isee ref. 12a.
- 14
- 14aT. Yanagi, S. Otsuka, Y. Kasuga, K. Fujimoto, K. Murakami, K. Nogi, H. Yorimitsu, A. Osuka, J. Am. Chem. Soc. 2016, 138, 14582;
- 14bZ. He, H. J. Shrives, J. A. Fernández-Salas, A. Abengózar, J. Neufeld, K. Yang, A. P. Pulis, D. J. Procter, Angew. Chem. Int. Ed. 2018, 57, 5759;
- 14cK. Yang, A. P. Pulis, G. J. P. Perry, D. J. Procter, Org. Lett. 2018, 20, 7498;
- 14dM. Hu, Y. Liu, Y. Liang, T. Dong, L. Kong, M. Bao, Z.-X. Wang, B. Peng, Nat. Commun. 2022, 13, 4719;
- 14eW. Zhao, X. Huang, Y. Zhan, Q. Zhang, D. Li, Y. Zhang, L. Kong, B. Peng, Angew. Chem. Int. Ed. 2019, 58, 17210;
- 14fsee ref. 5b;
- 14gH. J. Shrives, J. A. Fernández-Salas, C. Hedtke, A. P. Pulis, D. J. Procter, Nat. Commun. 2017, 8, 14801;
- 14hR. Bisht, M. V. Popescu, Z. He, A. M. Ibrahim, G. E. M. Crisenza, R. S. Paton, D. J. Procter, Angew. Chem. Int. Ed. 2023, 62, e202302418.
- 15
- 15aY. Macé, C. Urban, C. Pradet, J.-C. Blazejewski, E. Magnier, Eur. J. Org. Chem. 2009, 5313;
- 15bB. Pégot, C. Urban, P. Diter, E. Magnier, Eur. J. Org. Chem. 2013, 7800.
- 16
- 16aA. Nilova, L.-C. Campeau, E. C. Sherer, D. R. Stuart, J. Med. Chem. 2020, 63, 13389;
- 16bD. G. Brown, M. M. Gagnon, J. Boström, J. Med. Chem. 2015, 58, 2390;
- 16cA. Senior, K. Ruffell, L. T. Ball, Nat. Chem. 2023, 15, 386.
- 17
- 17aR. López, C. Palomo, Angew. Chem. Int. Ed. 2015, 54, 13170;
- 17bC. C. C. Johansson, T. J. Colacot, Angew. Chem. Int. Ed. 2010, 49, 676;
- 17cF. Bellina, R. Rossi, Chem. Rev. 2010, 110, 1082.
- 18
- 18asee ref. 17c;
- 18bX. Liu, J. F. Hartwig, J. Am. Chem. Soc. 2004, 126, 5182.
- 19In addition to 17 a, the sulfurized nucleophiles 17 b and 17 c were obtained in 50 % and 47 % yields from the reactions of 1 p/2 a/3 a and 1 g/2 a/3 d, respectively.