Atomically Precise Copper Nanoclusters for Highly Efficient Electroreduction of CO2 towards Hydrocarbons via Breaking the Coordination Symmetry of Cu Site
Qiu-Jin Wu
State Key Laboratory of Structural Chemistry, Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences Fujian, Fuzhou, 350002 P. R. China
University of Chinese Academy of Sciences, Beijing, 100049 P. R. China
These authors contributed equally to this work.
Search for more papers by this authorDr. Duan-Hui Si
State Key Laboratory of Structural Chemistry, Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences Fujian, Fuzhou, 350002 P. R. China
These authors contributed equally to this work.
Search for more papers by this authorProf. Pan-Pan Sun
Key Laboratory of Colloid and Interface Chemistry, Ministry of Education, School of Chemistry and Chemical Engi-neering, State Key Laboratory of Crystal Materials, Shandong University, Jinan, Shandong, 250100 P. R. China
Search for more papers by this authorYu-Liang Dong
State Key Laboratory of Structural Chemistry, Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences Fujian, Fuzhou, 350002 P. R. China
Search for more papers by this authorSong Zheng
State Key Laboratory of Structural Chemistry, Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences Fujian, Fuzhou, 350002 P. R. China
Search for more papers by this authorQian Chen
State Key Laboratory of Structural Chemistry, Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences Fujian, Fuzhou, 350002 P. R. China
Search for more papers by this authorShi-Hua Ye
State Key Laboratory of Structural Chemistry, Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences Fujian, Fuzhou, 350002 P. R. China
Search for more papers by this authorCorresponding Author
Prof. Di Sun
Key Laboratory of Colloid and Interface Chemistry, Ministry of Education, School of Chemistry and Chemical Engi-neering, State Key Laboratory of Crystal Materials, Shandong University, Jinan, Shandong, 250100 P. R. China
Search for more papers by this authorCorresponding Author
Prof. Rong Cao
State Key Laboratory of Structural Chemistry, Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences Fujian, Fuzhou, 350002 P. R. China
Fujian Science & Technology Innovation Laboratory for Optoelectronic Information of China, Fuzhou, Fujian, 350108 P. R. China
University of Chinese Academy of Sciences, Beijing, 100049 P. R. China
Search for more papers by this authorCorresponding Author
Prof. Yuan-Biao Huang
State Key Laboratory of Structural Chemistry, Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences Fujian, Fuzhou, 350002 P. R. China
Fujian Science & Technology Innovation Laboratory for Optoelectronic Information of China, Fuzhou, Fujian, 350108 P. R. China
University of Chinese Academy of Sciences, Beijing, 100049 P. R. China
Search for more papers by this authorQiu-Jin Wu
State Key Laboratory of Structural Chemistry, Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences Fujian, Fuzhou, 350002 P. R. China
University of Chinese Academy of Sciences, Beijing, 100049 P. R. China
These authors contributed equally to this work.
Search for more papers by this authorDr. Duan-Hui Si
State Key Laboratory of Structural Chemistry, Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences Fujian, Fuzhou, 350002 P. R. China
These authors contributed equally to this work.
Search for more papers by this authorProf. Pan-Pan Sun
Key Laboratory of Colloid and Interface Chemistry, Ministry of Education, School of Chemistry and Chemical Engi-neering, State Key Laboratory of Crystal Materials, Shandong University, Jinan, Shandong, 250100 P. R. China
Search for more papers by this authorYu-Liang Dong
State Key Laboratory of Structural Chemistry, Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences Fujian, Fuzhou, 350002 P. R. China
Search for more papers by this authorSong Zheng
State Key Laboratory of Structural Chemistry, Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences Fujian, Fuzhou, 350002 P. R. China
Search for more papers by this authorQian Chen
State Key Laboratory of Structural Chemistry, Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences Fujian, Fuzhou, 350002 P. R. China
Search for more papers by this authorShi-Hua Ye
State Key Laboratory of Structural Chemistry, Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences Fujian, Fuzhou, 350002 P. R. China
Search for more papers by this authorCorresponding Author
Prof. Di Sun
Key Laboratory of Colloid and Interface Chemistry, Ministry of Education, School of Chemistry and Chemical Engi-neering, State Key Laboratory of Crystal Materials, Shandong University, Jinan, Shandong, 250100 P. R. China
Search for more papers by this authorCorresponding Author
Prof. Rong Cao
State Key Laboratory of Structural Chemistry, Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences Fujian, Fuzhou, 350002 P. R. China
Fujian Science & Technology Innovation Laboratory for Optoelectronic Information of China, Fuzhou, Fujian, 350108 P. R. China
University of Chinese Academy of Sciences, Beijing, 100049 P. R. China
Search for more papers by this authorCorresponding Author
Prof. Yuan-Biao Huang
State Key Laboratory of Structural Chemistry, Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences Fujian, Fuzhou, 350002 P. R. China
Fujian Science & Technology Innovation Laboratory for Optoelectronic Information of China, Fuzhou, Fujian, 350108 P. R. China
University of Chinese Academy of Sciences, Beijing, 100049 P. R. China
Search for more papers by this authorGraphical Abstract
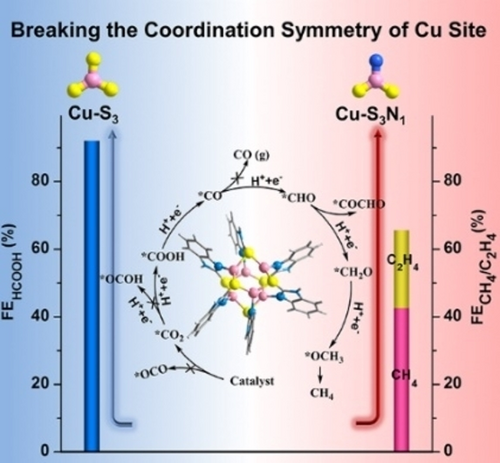
Breaking the coordination symmetry of Cu site in atomically precise Cu6 cluster forms Cu-S2N1 site, which rank the dx2-y2 orbital as the highest occupied d orbital to favor the specific coordination between C atom of CO2 and Cu−S2N1 site. This binding mode is conductive to the generation of *COOH instead of *OCHO, thereby switching the product of electrocatalytic CO2 reduction reaction to higher-valued hydrocarbons.
Abstract
We propose an effective highest occupied d-orbital modulation strategy engendered by breaking the coordination symmetry of sites in the atomically precise Cu nanocluster (NC) to switch the product of CO2 electroreduction from HCOOH/CO to higher-valued hydrocarbons. An atomically well-defined Cu6 NC with symmetry-broken Cu−S2N1 active sites (named Cu6(MBD)6, MBD=2-mercaptobenzimidazole) was designed and synthesized by a judicious choice of ligand containing both S and N coordination atoms. Different from the previously reported high HCOOH selectivity of Cu NCs with Cu−S3 sites, the Cu6(MBD)6 with Cu−S2N1 coordination structure shows a high Faradaic efficiency toward hydrocarbons of 65.5 % at −1.4 V versus the reversible hydrogen electrode (including 42.5 % CH4 and 23 % C2H4), with the hydrocarbons partial current density of −183.4 mA cm−2. Theoretical calculations reveal that the symmetry-broken Cu−S2N1 sites can rearrange the Cu 3d orbitals with 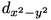 as the highest occupied d-orbital, thus favoring the generation of key intermediate *COOH instead of *OCHO to favor *CO formation, followed by hydrogenation and/or C−C coupling to produce hydrocarbons. This is the first attempt to regulate the coordination mode of Cu atom in Cu NCs for hydrocarbons generation, and provides new inspiration for designing atomically precise NCs for efficient CO2RR towards highly-valued products.
as the highest occupied d-orbital, thus favoring the generation of key intermediate *COOH instead of *OCHO to favor *CO formation, followed by hydrogenation and/or C−C coupling to produce hydrocarbons. This is the first attempt to regulate the coordination mode of Cu atom in Cu NCs for hydrocarbons generation, and provides new inspiration for designing atomically precise NCs for efficient CO2RR towards highly-valued products.
Conflict of interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202306822-sup-0001-Cu6(MBD)6.cif367.6 KB | Supporting Information |
| anie202306822-sup-0001-misc_information.pdf3.5 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aY. Yang, S. Louisia, S. Yu, J. Jin, I. Roh, C. Chen, M. V. Fonseca Guzman, J. Feijoo, P. C. Chen, H. Wang, C. J. Pollock, X. Huang, Y. T. Shao, C. Wang, D. A. Muller, H. D. Abruna, P. Yang, Nature 2023, 614, 262–269;
- 1bF. Franco, C. Rettenmaier, H. S. Jeon, B. Roldan Cuenya, Chem. Soc. Rev. 2020, 49, 6884–6946;
- 1cS. Kong, X. Lv, X. Wang, Z. Liu, Z. Li, B. Jia, D. Sun, C. Yang, L. Liu, A. Guan, J. Wang, G. Zheng, F. Huang, Nat. Catal. 2022, 6, 6–15;
- 1dQ. J. Wu, J. Liang, Y. B. Huang, R. Cao, Acc. Chem. Res. 2022, 55, 2978–2997;
- 1eN. Li, D.-H. Si, Q.-J. Wu, Q. Wu, Y.-B. Huang, R. Cao, CCS Chem. 2023, 5, 1130–1143;
- 1fY. Hou, Y.-B. Huang, Y.-L. Liang, G.-L. Chai, J.-D. Yi, T. Zhang, K.-T. Zang, J. Luo, R. Xu, H. Lin, S.-Y. Zhang, H.-M. Wang, R. Cao, CCS Chem. 2019, 1, 384–395.
- 2
- 2aL. Sun, V. Reddu, A. C. Fisher, X. Wang, Energy Environ. Sci. 2020, 13, 374–403;
- 2bM. G. Kibria, C. T. Dinh, A. Seifitokaldani, P. De Luna, T. Burdyny, R. Quintero-Bermudez, M. B. Ross, O. S. Bushuyev, F. P. Garcia de Arquer, P. Yang, D. Sinton, E. H. Sargent, Adv. Mater. 2018, 30, 1804867;
- 2cS. Ajmal, G. Yasin, A. Kumar, M. Tabish, S. Ibraheem, K. A. Sammed, M. A. Mushtaq, A. Saad, Z. Mo, W. Zhao, Coord. Chem. Rev. 2023, 485, 215099.
- 3
- 3aJ. D. Yi, R. Xie, Z. L. Xie, G. L. Chai, T. F. Liu, R. P. Chen, Y. B. Huang, R. Cao, Angew. Chem. Int. Ed. 2020, 59, 23641–23648;
- 3bL. Zaza, K. Rossi, R. Buonsanti, ACS Energy Lett. 2022, 7, 1284–1291;
- 3cD. L. Meng, M. D. Zhang, D. H. Si, M. J. Mao, Y. Hou, Y. B. Huang, R. Cao, Angew. Chem. Int. Ed. 2021, 60, 25485–25492;
- 3dA. Loiudice, P. Lobaccaro, E. A. Kamali, T. Thao, B. H. Huang, J. W. Ager, R. Buonsanti, Angew. Chem. Int. Ed. 2016, 55, 5789–5792;
- 3eZ. Z. Wu, X. L. Zhang, Z. Z. Niu, F. Y. Gao, P. P. Yang, L. P. Chi, L. Shi, W. S. Wei, R. Liu, Z. Chen, S. Hu, X. Zheng, M. R. Gao, J. Am. Chem. Soc. 2022, 144, 259–269;
- 3fG. L. De Gregorio, T. Burdyny, A. Loiudice, P. Iyengar, W. A. Smith, R. Buonsanti, ACS Catal. 2020, 10, 4854–4862;
- 3gZ. Gu, H. Shen, Z. Chen, Y. Yang, C. Yang, Y. Ji, Y. Wang, C. Zhu, J. Liu, J. Li, T.-K. Sham, X. Xu, G. Zheng, Joule 2021, 5, 429–440;
- 3hC. P. Wan, J. D. Yi, R. Cao, Y. B. Huang, Chin. J. Struct. Chem. 2022, 41, 2205001–2205014.
- 4
- 4aL.-J. Zhu, D.-H. Si, F.-X. Ma, M.-J. Sun, T. Zhang, R. Cao, ACS Catal. 2023, 13, 5114–5121;
- 4bR. Wang, J. Liu, L.-Z. Dong, J. Zhou, Q. Huang, Y.-R. Wang, J.-W. Shi, Y.-Q. Lan, CCS Chem. 2023, https://doi.org/10.31635/ccschem.022.202202316.
10.31635/ccschem.022.202202316 Google Scholar
- 5
- 5aH.-L. Zhu, J.-R. Huang, X.-W. Zhang, C. Wang, N.-Y. Huang, P.-Q. Liao, X.-M. Chen, ACS Catal. 2021, 11, 11786–11792;
- 5bJ. Liu, D. Yang, Y. Zhou, G. Zhang, G. Xing, Y. Liu, Y. Ma, O. Terasaki, S. Yang, L. Chen, Angew. Chem. Int. Ed. 2021, 60, 14473–14479.
- 6
- 6aL. Li, Y. F. Sun, Y. Xie, Natl. Sci. Rev. 2023, 10, nwac230;
- 6bS. Overa, B. H. Ko, Y. Zhao, F. Jiao, Acc. Chem. Res. 2022, 55, 638–648;
- 6cX. Lv, Z. Liu, C. Yang, Y. Ji, G. Zheng, Acc. Mater. Res. 2023, 4, 264–274;
- 6dC. Peng, G. Luo, J. Zhang, M. Chen, Z. Wang, T. K. Sham, L. Zhang, Y. Li, G. Zheng, Nat. Commun. 2021, 12, 1580.
- 7
- 7aY. Yang, J.-J. Fu, Y.-X. Ouyang, T. Tang, Y. Zhang, L.-R. Zheng, Q.-H. Zhang, X.-Z. Liu, J.-L. Wang, J.-S. Hu, Natl. Sci. Rev. 2022, 10, nwac248;
- 7bX. Su, Z. Jiang, J. Zhou, H. Liu, D. Zhou, H. Shang, X. Ni, Z. Peng, F. Yang, W. Chen, Z. Qi, D. Wang, Y. Wang, Nat. Commun. 2022, 13, 1322.
- 8Y. Xu, F. Li, A. Xu, J. P. Edwards, S. F. Hung, C. M. Gabardo, C. P. O′Brien, S. Liu, X. Wang, Y. Li, J. Wicks, R. K. Miao, Y. Liu, J. Li, J. E. Huang, J. Abed, Y. Wang, E. H. Sargent, D. Sinton, Nat. Commun. 2021, 12, 2932.
- 9
- 9aX. Cai, G. Li, W. Hu, Y. Zhu, ACS Catal. 2022, 12, 10638–10653;
- 9bS. Li, A. V. Nagarajan, X. Du, Y. Li, Z. Liu, D. R. Kauffman, G. Mpourmpakis, R. Jin, Angew. Chem. Int. Ed. 2022, 61, e202211771;
- 9cY. Yang, T. Jia, Y. Z. Han, Z. A. Nan, S. F. Yuan, F. L. Yang, D. Sun, Angew. Chem. Int. Ed. 2019, 58, 12280–12285;
- 9dQ. Tang, Y. Lee, D. Y. Li, W. Choi, C. W. Liu, D. Lee, D. E. Jiang, J. Am. Chem. Soc. 2017, 139, 9728–9736;
- 9eL. J. Liu, Z. Y. Wang, Z. Y. Wang, R. Wang, S. Q. Zang, T. C. W. Mak, Angew. Chem. Int. Ed. 2022, 61, e202205626;
- 9fF. Li, Q. Tang, J. Catal. 2020, 387, 95–101.
- 10Q. Tang, G. Hu, V. Fung, D. E. Jiang, Acc. Chem. Res. 2018, 51, 2793–2802.
- 11J. Wang, F. Xu, Z. Y. Wang, S. Q. Zang, T. C. W. Mak, Angew. Chem. Int. Ed. 2022, 61, e202207492.
- 12G. Deng, J. Kim, M. S. Bootharaju, F. Sun, K. Lee, Q. Tang, Y. J. Hwang, T. Hyeon, J. Am. Chem. Soc. 2023, 145, 3401–3407.
- 13
- 13aT. K. Todorova, M. W. Schreiber, M. Fontecave, ACS Catal. 2020, 10, 1754–1768;
- 13bT. Shinagawa, G. O. Larrazábal, A. J. Martín, F. Krumeich, J. Pérez-Ramírez, ACS Catal. 2018, 8, 837–844.
- 14
- 14aT. Zhang, Z. Sun, S. Li, B. Wang, Y. Liu, R. Zhang, Z. Zhao, Nat. Commun. 2022, 13, 6996;
- 14bF. Wang, R. Zhang, Y. Zhang, Y. Li, J. Zhang, W. Yuan, H. Liu, F. Wang, H. L. Xin, Adv. Funct. Mater. 2023, 33, 2213863;
- 14cH. Kim, D. Shin, W. Yang, D. H. Won, H. S. Oh, M. W. Chung, D. Jeong, S. H. Kim, K. H. Chae, J. Y. Ryu, J. Lee, S. J. Cho, J. Seo, H. Kim, C. H. Choi, J. Am. Chem. Soc. 2021, 143, 925–933;
- 14dY. Jia, Z. Xue, J. Yang, Q. Liu, J. Xian, Y. Zhong, Y. Sun, X. Zhang, Q. Liu, D. Yao, G. Li, Angew. Chem. Int. Ed. 2022, 61, e202110838;
- 14eX. F. Cheng, J. H. He, H. Q. Ji, H. Y. Zhang, Q. Cao, W. J. Sun, C. L. Yan, J. M. Lu, Adv. Mater. 2022, 34, 2205767.
- 15J. Zhao, P. Zhang, T. Yuan, D. Cheng, S. Zhen, H. Gao, T. Wang, Z. J. Zhao, J. Gong, J. Am. Chem. Soc. 2023, 145, 6622–6627.
- 16Deposition number 2259405 (for Cu6(MBD)6 at 200 K) contains the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service.
- 17L. Cao, Q. Luo, W. Liu, Y. Lin, X. Liu, Y. Cao, W. Zhang, Y. Wu, J. Yang, T. Yao, S. Wei, Nat. Catal. 2018, 2, 134–141.
- 18H. B. Yang, S.-F. Hung, S. Liu, K. Yuan, S. Miao, L. Zhang, X. Huang, H.-Y. Wang, W. Cai, R. Chen, J. Gao, X. Yang, W. Chen, Y. Huang, H. M. Chen, C. M. Li, T. Zhang, B. Liu, Nat. Energy 2018, 3, 140–147.
- 19H. Li, J. Zhao, L. Luo, J. Du, J. Zeng, Acc. Chem. Res. 2021, 54, 1454–1464.
- 20
- 20aK. Jiang, K. Xu, S. Zou, W. B. Cai, J. Am. Chem. Soc. 2014, 136, 4861–4864;
- 20bX. Q. Duan, G. Y. Duan, Y. F. Wang, X. Q. Li, R. Wang, R. Zhang, B. H. Xu, Small 2023, 19, 2207219.
- 21G. Zhao, G. Hai, P. Zhou, Z. Liu, Y. Zhang, B. Peng, W. Xia, X. Huang, G. Wang, Adv. Funct. Mater. 2023, 33, 2213170.
- 22
- 22aS. Li, H. Duan, J. Yu, C. Qiu, R. Yu, Y. Chen, Y. Fang, X. Cai, S. Yang, ACS Catal. 2022, 12, 9074–9082;
- 22bW. Guo, X. Tan, J. Bi, L. Xu, D. Yang, C. Chen, Q. Zhu, J. Ma, A. Tayal, J. Huang, X. Sun, S. Liu, B. Han, J. Am. Chem. Soc. 2021, 143, 6877–6885.