Pathway Control of π-Conjugated Supramolecular Polymers by Incorporating Donor-Acceptor Functionality
Fan Wang
Key Laboratory of Precision and Intelligent Chemistry, Department of Polymer Science and Engineering, University of Science and Technology of China, Hefei, Anhui, 230026 P. R. China
These authors contributed equally to this work.
Search for more papers by this authorRui Liao
Key Laboratory of Precision and Intelligent Chemistry, Department of Polymer Science and Engineering, University of Science and Technology of China, Hefei, Anhui, 230026 P. R. China
These authors contributed equally to this work.
Search for more papers by this authorCorresponding Author
Prof. Dr. Feng Wang
Key Laboratory of Precision and Intelligent Chemistry, Department of Polymer Science and Engineering, University of Science and Technology of China, Hefei, Anhui, 230026 P. R. China
Search for more papers by this authorFan Wang
Key Laboratory of Precision and Intelligent Chemistry, Department of Polymer Science and Engineering, University of Science and Technology of China, Hefei, Anhui, 230026 P. R. China
These authors contributed equally to this work.
Search for more papers by this authorRui Liao
Key Laboratory of Precision and Intelligent Chemistry, Department of Polymer Science and Engineering, University of Science and Technology of China, Hefei, Anhui, 230026 P. R. China
These authors contributed equally to this work.
Search for more papers by this authorCorresponding Author
Prof. Dr. Feng Wang
Key Laboratory of Precision and Intelligent Chemistry, Department of Polymer Science and Engineering, University of Science and Technology of China, Hefei, Anhui, 230026 P. R. China
Search for more papers by this authorGraphical Abstract
Abstract
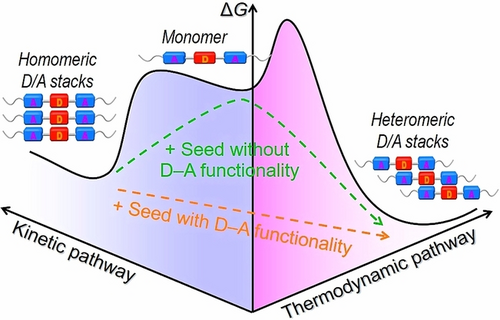
Controlling the nanoscale orientation of π-conjugated systems remains challenging due to the complexity of multiple energy landscapes involved in the supramolecular assembly process. In this study, we have developed an effective strategy for programming the pathways of π-conjugated supramolecular polymers, by incorporating both electron-rich methoxy- or methanthiol-benzene as donor unit and electron-poor cyano-vinylenes as acceptor units on the monomeric structure. It leads to the formation of parallel-stacked supramolecular polymers as the metastable species through homomeric donor/acceptor packing, which convert to slip-stacked supramolecular polymers as the thermodynamically stable species facilitated by heteromeric donor-acceptor packing. By further investigating the external seed-induced kinetic-to-thermodynamic transformation behaviors, our findings suggest that the donor-acceptor functionality on the seed structure is crucial for accelerating pathway conversion. This is achieved by eliminating the initial lag phase in the supramolecular polymerization process. Overall, this study provides valuable insights into designing molecular structures that control aggregation pathways of π-conjugated nanostructures.
Conflict of interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202305827-sup-0001-misc_information.pdf4.7 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1F. J. M. Hoeben, P. Jonkheijm, E. W. Meijer, A. P. H. J. Schenning, Chem. Rev. 2005, 105, 1491–1546.
- 2
- 2aP. A. Korevaar, T. F. A. de Greef, E. W. Meijer, Chem. Mater. 2014, 26, 576–586;
- 2bA. Sorrenti, J. L. Iglesias, A. J. Markvoort, T. F. A. de Greef, T. M. Hermans, Chem. Soc. Rev. 2017, 46, 5476–5490;
- 2cZ. Huang, B. Qin, L. Chen, J. Xu, C. F. J. Faul, X. Zhang, Macromol. Rapid Commun. 2017, 38, 1700312;
- 2dM. Hartlieb, E. D. H. Mansfield, S. Perrier, Polym. Chem. 2020, 11, 1083–1110.
- 3
- 3aP. A. Korevaar, S. J. George, A. J. Markvoort, M. M. J. Smulders, P. A. J. Hilbers, A. P. H. J. Schenning, T. F. A. de Greef, E. W. Meijer, Nature 2012, 481, 492–496;
- 3bJ. Matern, Y. Dorca, L. Sánchez, G. Fernández, Angew. Chem. Int. Ed. 2019, 58, 16730–16740;
- 3cM. Wehner, F. Würthner, Nat. Chem. Rev. 2020, 4, 38–53;
- 3dZ. Gao, Y. Tian, H.-K. Hsu, Y. Han, Y.-T. Chan, F. Wang, CCS Chem. 2021, 3, 105–115.
- 4
- 4aS. Ogi, K. Sugiyasu, S. Manna, S. Samitsu, M. Takeuchi, Nat. Chem. 2014, 6, 188–195;
- 4bJ. Kang, D. Miyajima, T. Mori, Y. Inoue, Y. Itoh, T. Aida, Science 2015, 347, 646–651;
- 4cS. H. Jung, D. Bochicchio, G. M. Pavan, M. Takeuchi, K. Sugiyasu, J. Am. Chem. Soc. 2018, 140, 10570–10577;
- 4dS. Sarkar, A. Sarkar, S. J. George, Angew. Chem. Int. Ed. 2020, 59, 19841–19845.
- 5
- 5aS. Ogi, V. Stepanenko, K. Sugiyasu, M. Takeuchi, F. Würthner, J. Am. Chem. Soc. 2015, 137, 3300–3307;
- 5bS. Ogi, V. Stepanenko, J. Thein, F. Würthner, J. Am. Chem. Soc. 2016, 138, 670–678;
- 5cJ. S. Valera, R. Gómez, L. Sánchez, Small 2018, 14, 1702437;
- 5dI. Helmers, G. Ghosh, R. Q. Albuquerque, G. Fernández, Angew. Chem. Int. Ed. 2021, 60, 4368–4376;
- 5eC. Naranjo, S. Adalid, R. Gómez, L. Sánchez, Angew. Chem. Int. Ed. 2023, 62, e202218572.
- 6
- 6aA. Langenstroer, K. K. Kartha, Y. Dorca, J. Droste, V. Stepanenko, R. Q. Albuquerque, M. R. Hansen, L. Sánchez, G. Fernández, J. Am. Chem. Soc. 2019, 141, 5192–5200;
- 6bJ. Matern, N. Bäumer, G. Fernández, J. Am. Chem. Soc. 2021, 143, 7164–7175.
- 7
- 7aA. Aliprandi, M. Mauro, L. de Cola, Nat. Chem. 2016, 8, 10–15;
- 7bK. Zhang, M. C. Yeung, S. Y. Leung, V. W. W. Yam, J. Am. Chem. Soc. 2018, 140, 9594–9605;
- 7cJ. Matern, I. Maisuls, C. A. Strassert, G. Fernández, Angew. Chem. Int. Ed. 2022, 61, e202208436.
- 8
- 8aS. S. Babu, V. K. Praveen, S. Prasanthkumar, A. Ajayaghosh, Chem. Eur. J. 2008, 14, 9577–9584;
- 8bK. V. Rao, K. Jayaramulu, T. K. Maji, S. J. George, Angew. Chem. Int. Ed. 2010, 49, 4218–4222;
- 8cR. Liao, F. Wang, Y. Guo, Y. Han, F. Wang, J. Am. Chem. Soc. 2022, 144, 9775–9784;
- 8dD. Jia, H. Zhong, S. Jiang, R. Yao, F. Wang, Chin. Chem. Lett. 2022, 33, 4900–4903.
- 9
- 9aW.-S. Li, Y. Yamamoto, T. Fukushima, A. Saeki, S. Seki, S. Tagawa, H. Masunaga, S. Sasaki, M. Takata, T. Aida, J. Am. Chem. Soc. 2008, 130, 8886–8887;
- 9bM. R. Molla, A. Dasa, S. Ghosh, Chem. Commun. 2011, 47, 8934–8936;
- 9cB. Narayan, K. K. Bejagam, S. Balasubramanian, S. J. George, Angew. Chem. Int. Ed. 2015, 54, 13053–13057;
- 9dA. Mishra, D. B. Korlepara, M. Kumar, A. Jain, N. Jonnalagadda, K. K. Bejagam, S. Balasubramanian, S. J. George, Nat. Commun. 2018, 9, 1295.
- 10
- 10aZ. Wang, Q. Zhang, Asian J. Org. Chem. 2020, 9, 1252–1261;
- 10bW. Wang, L. Luo, P. Sheng, J. Zhang, Q. Zhang, Chem. Eur. J. 2021, 27, 464–490.
- 11
- 11aB. An, J. Gierschner, S. Y. Park, Acc. Chem. Res. 2012, 45, 544–554;
- 11bM. Martínez-Abadía, R. Giménez, M. B. Ros, Adv. Mater. 2018, 30, 1704161.
- 12
- 12aT. F. A. de Greef, M. M. J. Smulders, M. Wolffs, A. P. H. J. Schenning, R. P. Sijbesma, E. W. Meijer, Chem. Rev. 2009, 109, 5687–5754;
- 12bJ. Chen, L. Ao, C. Wei, C. Wang, F. Wang, Chem. Commun. 2019, 55, 229–232;
- 12cZ. Gao, Z. Chen, Y. Han, F. Wang, Nanoscale Horiz. 2020, 5, 1081–1087;
- 12dY. Han, Y. Yin, F. Wang, F. Wang, Angew. Chem. Int. Ed. 2021, 60, 14076–14082;
- 12eY. Han, X. Zhang, Z. Ge, Z. Gao, R. Liao, F. Wang, Nat. Commun. 2022, 13, 3546.
- 13
- 13aH. Choi, S. Ogi, N. Ando, S. Yamaguchi, J. Am. Chem. Soc. 2021, 143, 2953–2961;
- 13bO. Shyshov, S. Vadakket Haridas, L. Pesce, H. Qi, A. Gardin, D. Bochicchio, U. Kaiser, G. M. Pavan, M. von Delius, Nat. Commun. 2021, 12, 3134.
- 14
- 14aJ. H. Kim, J. W. Chung, Y. Jung, S. Yoon, B. K. An, H. S. Huh, S. W. Leec, S. Y. Park, J. Mater. Chem. 2010, 20, 10103–10106;
- 14bY. Xue, S. Jiang, H. Zhong, Z. Chen, F. Wang, Angew. Chem. Int. Ed. 2022, 61, e202110766;
- 14cL. López-Gandul, C. Naranjo, C. Sanchez, R. Rodriguez, R. Gomez, J. Crassous, L. Sanchez, Chem. Sci. 2022, 13, 11577–11584;
- 14dY. Xue, C. Zhang, T. Lv, L. Qiu, F. Wang, Angew. Chem. Int. Ed. 2023, 62, e202300972.
- 15
- 15aB. Adelizzi, A. Aloi, A. J. Markvoort, H. M. M. ten Eikelder, I. K. Voets, A. R. A. Palmans, E. W. Meijer, J. Am. Chem. Soc. 2018, 140, 7168–7175;
- 15bT. Schnitzer, S. A. H. Jansen, M. F. J. Mabesoone, G. Vantomme, E. W. Meijer, Angew. Chem. Int. Ed. 2022, 61, e202206729.
- 16J. Shi, L. E. A. Suarez, S. J. Yoon, S. Varghese, C. Serpa, S. Y. Park, L. Lüer, D. R. Sanjuan, B. M. Medina, J. Gierschner, J. Phys. Chem. C 2017, 121, 23166–23183.
- 17
- 17aM. M. J. Smulders, M. M. L. Nieuwenhuizen, T. F. A. de Greef, P. van der Schoot, A. P. H. J. Schenning, E. W. Meijer, Chem. Eur. J. 2010, 16, 362–367;
- 17bA. Isobe, D. D. Prabhu, S. Datta, T. Aizawa, S. Yagai, Chem. Eur. J. 2020, 26, 8997–9004.
- 18
- 18aL. Bentea, M. A. Watzky, R. G. Finke, J. Phys. Chem. C 2017, 121, 5302–5312;
- 18bA. Sarkar, R. Sasmal, A. Das, A. Venugopal, S. S. Agasti, S. J. George, Angew. Chem. Int. Ed. 2021, 60, 18209–18216.
- 19E. E. Greciano, J. Calbo, E. Ortí, L. Sánchez, Angew. Chem. Int. Ed. 2020, 59, 17517–17524.
- 20G. Odian, Principles of Polymerization, Wiley, Hoboken, 2004, pp. 313–319.
10.1002/047147875X Google Scholar
- 21B. Adelizzi, A. Aloi, A. J. Markvoort, H. M. M. T. Eikelder, I. K. Voets, A. R. A. Palmans, E. W. Meijer, J. Am. Chem. Soc. 2018, 140, 7168–7175.
- 22W. Wagner, M. Wehner, V. Stepanenko, F. Würthner, CCS Chem. 2019, 1, 598–613.
- 23
- 23aS. Datta, Y. Kato, S. Higashiharaguchi, K. Aratsu, A. Isobe, T. Saito, D. D. Prabhu, Y. Kitamoto, M. J. Hollamby, A. J. Smith, R. Dalgliesh, N. Mahmoudi, L. Pesce, C. Perego, G. M. Pavan, S. Yagai, Nature 2020, 583, 400–405;
- 23bR. Laishram, S. Sarkar, I. Seth, N. Khatun, V. K. Aswal, U. Maitra, S. J. George, J. Am. Chem. Soc. 2022, 144, 11306–11315.