Cl2⋅− Mediates Direct and Selective Conversion of Inert C(sp3)−H Bonds into Aldehydes/Ketones
Dr. Qinhua Zhang
State Key Laboratory of Heavy Oil Processing, Institute of New Energy, College of Chemical Engineering, China University of Petroleum (East China), Qingdao, 266580 P. R. China
Contribution: Data curation (lead), Investigation (lead), Methodology (lead), Software (lead), Writing - original draft (lead)
Search for more papers by this authorDr. Bo An
State Key Laboratory of Heavy Oil Processing, Institute of New Energy, College of Chemical Engineering, China University of Petroleum (East China), Qingdao, 266580 P. R. China
Contribution: Data curation (supporting), Software (supporting)
Search for more papers by this authorDr. Yu Lei
Guangdong Provincial Key Laboratory of Environmental Pollution Control and Remediation Technology, School of Environmental Science and Engineering, Sun Yat-sen University, Guangzhou, 510275 China
Contribution: Data curation (supporting), Investigation (supporting)
Search for more papers by this authorZhixiao Gao
State Key Laboratory of Heavy Oil Processing, Institute of New Energy, College of Chemical Engineering, China University of Petroleum (East China), Qingdao, 266580 P. R. China
Contribution: Resources (supporting), Software (supporting)
Search for more papers by this authorDr. Haonan Zhang
State Key Laboratory of Heavy Oil Processing, Institute of New Energy, College of Chemical Engineering, China University of Petroleum (East China), Qingdao, 266580 P. R. China
Contribution: Data curation (supporting), Validation (supporting)
Search for more papers by this authorProf. Sheng Xue
Institute for Translational Medicine, The Affiliated Hospital of Qingdao University, College of Medicine, Qingdao University, Qingdao, 266021 P. R. China
Contribution: Conceptualization (supporting), Resources (supporting)
Search for more papers by this authorProf. Xin Jin
State Key Laboratory of Heavy Oil Processing, Institute of New Energy, College of Chemical Engineering, China University of Petroleum (East China), Qingdao, 266580 P. R. China
Contribution: Formal analysis (supporting), Resources (supporting)
Search for more papers by this authorProf. Wengang Xu
State Key Laboratory of Heavy Oil Processing, Institute of New Energy, College of Chemical Engineering, China University of Petroleum (East China), Qingdao, 266580 P. R. China
Contribution: Investigation (supporting), Methodology (supporting)
Search for more papers by this authorZihan Wu
State Key Laboratory of Heavy Oil Processing, Institute of New Energy, College of Chemical Engineering, China University of Petroleum (East China), Qingdao, 266580 P. R. China
Contribution: Data curation (supporting)
Search for more papers by this authorCorresponding Author
Prof. Mingbo Wu
State Key Laboratory of Heavy Oil Processing, Institute of New Energy, College of Chemical Engineering, China University of Petroleum (East China), Qingdao, 266580 P. R. China
Contribution: Funding acquisition (lead), Project administration (lead), Supervision (lead), Writing - review & editing (lead)
Search for more papers by this authorCorresponding Author
Prof. Xin Yang
Guangdong Provincial Key Laboratory of Environmental Pollution Control and Remediation Technology, School of Environmental Science and Engineering, Sun Yat-sen University, Guangzhou, 510275 China
Contribution: Investigation (equal), Methodology (equal), Writing - review & editing (equal)
Search for more papers by this authorCorresponding Author
Prof. Wenting Wu
State Key Laboratory of Heavy Oil Processing, Institute of New Energy, College of Chemical Engineering, China University of Petroleum (East China), Qingdao, 266580 P. R. China
Contribution: Formal analysis (lead), Project administration (lead), Supervision (lead), Writing - review & editing (lead)
Search for more papers by this authorDr. Qinhua Zhang
State Key Laboratory of Heavy Oil Processing, Institute of New Energy, College of Chemical Engineering, China University of Petroleum (East China), Qingdao, 266580 P. R. China
Contribution: Data curation (lead), Investigation (lead), Methodology (lead), Software (lead), Writing - original draft (lead)
Search for more papers by this authorDr. Bo An
State Key Laboratory of Heavy Oil Processing, Institute of New Energy, College of Chemical Engineering, China University of Petroleum (East China), Qingdao, 266580 P. R. China
Contribution: Data curation (supporting), Software (supporting)
Search for more papers by this authorDr. Yu Lei
Guangdong Provincial Key Laboratory of Environmental Pollution Control and Remediation Technology, School of Environmental Science and Engineering, Sun Yat-sen University, Guangzhou, 510275 China
Contribution: Data curation (supporting), Investigation (supporting)
Search for more papers by this authorZhixiao Gao
State Key Laboratory of Heavy Oil Processing, Institute of New Energy, College of Chemical Engineering, China University of Petroleum (East China), Qingdao, 266580 P. R. China
Contribution: Resources (supporting), Software (supporting)
Search for more papers by this authorDr. Haonan Zhang
State Key Laboratory of Heavy Oil Processing, Institute of New Energy, College of Chemical Engineering, China University of Petroleum (East China), Qingdao, 266580 P. R. China
Contribution: Data curation (supporting), Validation (supporting)
Search for more papers by this authorProf. Sheng Xue
Institute for Translational Medicine, The Affiliated Hospital of Qingdao University, College of Medicine, Qingdao University, Qingdao, 266021 P. R. China
Contribution: Conceptualization (supporting), Resources (supporting)
Search for more papers by this authorProf. Xin Jin
State Key Laboratory of Heavy Oil Processing, Institute of New Energy, College of Chemical Engineering, China University of Petroleum (East China), Qingdao, 266580 P. R. China
Contribution: Formal analysis (supporting), Resources (supporting)
Search for more papers by this authorProf. Wengang Xu
State Key Laboratory of Heavy Oil Processing, Institute of New Energy, College of Chemical Engineering, China University of Petroleum (East China), Qingdao, 266580 P. R. China
Contribution: Investigation (supporting), Methodology (supporting)
Search for more papers by this authorZihan Wu
State Key Laboratory of Heavy Oil Processing, Institute of New Energy, College of Chemical Engineering, China University of Petroleum (East China), Qingdao, 266580 P. R. China
Contribution: Data curation (supporting)
Search for more papers by this authorCorresponding Author
Prof. Mingbo Wu
State Key Laboratory of Heavy Oil Processing, Institute of New Energy, College of Chemical Engineering, China University of Petroleum (East China), Qingdao, 266580 P. R. China
Contribution: Funding acquisition (lead), Project administration (lead), Supervision (lead), Writing - review & editing (lead)
Search for more papers by this authorCorresponding Author
Prof. Xin Yang
Guangdong Provincial Key Laboratory of Environmental Pollution Control and Remediation Technology, School of Environmental Science and Engineering, Sun Yat-sen University, Guangzhou, 510275 China
Contribution: Investigation (equal), Methodology (equal), Writing - review & editing (equal)
Search for more papers by this authorCorresponding Author
Prof. Wenting Wu
State Key Laboratory of Heavy Oil Processing, Institute of New Energy, College of Chemical Engineering, China University of Petroleum (East China), Qingdao, 266580 P. R. China
Contribution: Formal analysis (lead), Project administration (lead), Supervision (lead), Writing - review & editing (lead)
Search for more papers by this authorGraphical Abstract
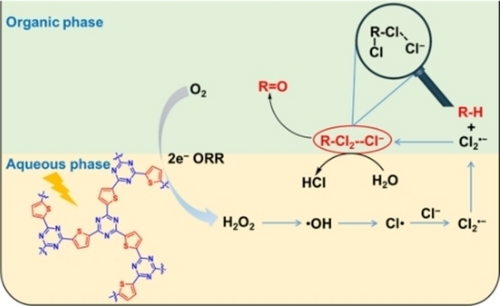
A thiophene-based covalent triazine polymer facilitates effective conversion of O2→H2O2→⋅OH→Cl⋅→Cl2.− in the aqueous phase. Cl2.−, with a longer lifetime, transfers into organic phase and activates C(sp3)−H bonds to generate unstable dichlorinated intermediates by successive hydrogen abstraction and chlorination. These unstable intermediates hydrolyze into either aldehydes or ketones with higher selectivity than that of benzyl dichloride.
Abstract
Developing new reactive pathway to activate inert C(sp3)−H bonds for valuable oxygenated products remains a challenge. We prepared a series of triazine conjugated organic polymers to photoactivate C−H into aldehyde/ketone via O2→H2O2→⋅OH→Cl⋅→Cl2⋅−. Experiment results showed Cl2⋅− could successively activate C(sp3)−H more effectively than Cl⋅ to generate unstable dichlorinated intermediates, increasing the kinetic rate ratio of dichlorination to monochlorination by a factor of 2,000 and thus breaking traditional dichlorination kinetic constraints. These active intermediates were hydrolyzed into aldehydes or ketones easily, when compared with typical stable dichlorinated complexes, avoiding chlorinated by-product generation. Moreover, an integrated two-phase system in an acid solution strengthened the Cl2⋅− mediated process and inhibited product overoxidation, where the conversion rate of toluene reached 16.94 mmol/g/h and the selectivity of benzaldehyde was 99.5 %. This work presents a facile and efficient approach for selective conversion of inert C(sp3)−H bonds using Cl2⋅−.
Conflict of interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202304699-sup-0001-misc_information.pdf2.1 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aR. G. Bergman, Nature 2007, 446, 391–393;
- 1bX. Cao, Z. Chen, R. Lin, W.-C. Cheong, S. Liu, J. Zhang, Q. Peng, C. Chen, T. Han, X. Tong, Y. Wang, R. Shen, W. Zhu, D. Wang, Y. Li, Nat. Catal. 2018, 1, 704–710;
- 1cD. I. Enache, J. K. Edwards, P. Landon, B. Solsona-Espriu, A. F. Carley, A. A. Herzing, M. Watanabe, C. J. Kiely, D. W. Knight, G. J. Hutchings, Science 2006, 311, 362–365.
- 2
- 2aG. Qi, T. E. Davies, A. Nasrallah, M. A. Sainna, A. G. R. Howe, R. J. Lewis, M. Quesne, C. R. A. Catlow, D. J. Willock, Q. He, D. Bethell, M. J. Howard, B. A. Murrer, B. Harrison, C. J. Kiely, X. Zhao, F. Deng, J. Xu, G. J. Hutchings, Nat. Catal. 2022, 5, 45–54;
- 2bV. L. Sushkevich, D. Palagin, M. Ranocchiari, J. A. van Bokhoven, Science 2017, 356, 523–527.
- 3
- 3aX. Wang, X. Yang, C. Zhao, Y. Pi, X. Li, Z. Jia, S. Zhou, J. Zhao, L. Wu, J. Liu, Angew. Chem. Int. Ed. 2023, 62, e202302829;
- 3bW. Wang, Q. Song, Q. Luo, L. Li, X. Huo, S. Chen, J. Li, Y. Li, S. Shi, Y. Yuan, X. Du, K. Zhang, N. Wang, Nat. Commun. 2023, 14, 2493;
- 3cT. Yang, Y. Jin, Y. Wang, A. Kong, Y. Chen, Y. Zou, C. Liu, G. Wei, C. Yu, Adv. Funct. Mater. 2023, 2300714;
- 3dK. Li, Q. Ge, Y. Liu, L. Wang, K. Gong, J. Liu, L. Xie, W. Wang, X. Ruan, L. Zhang, Energy Environ. Sci. 2023, 16, 1135–1145.
- 4Y. Zhou, L. Zhang, W. Wang, Nat. Commun. 2019, 10, 506.
- 5
- 5aC. Xu, Y. Pan, G. Wan, H. Liu, L. Wang, H. Zhou, S.-H. Yu, H.-L. Jiang, J. Am. Chem. Soc. 2019, 141, 19110–19117;
- 5bB. An, Q.-H. Zhang, B.-S. Zheng, M. Li, Y.-Y. Xi, X. Jin, S. Xue, Z.-T. Li, M.-B. Wu, W.-T. Wu, Angew. Chem. Int. Ed. 2022, 61, e202204661.
- 6K. Zhang, K. M. Parker, Environ. Sci. Technol. 2018, 52, 9579–9594.
- 7
- 7aS. M. Treacy, T. Rovis, J. Am. Chem. Soc. 2021, 143, 2729–2735;
- 7bG. Laudadio, S. Govaerts, Y. Wang, D. Ravelli, H. F. Koolman, M. Fagnoni, S. W. Djuric, T. Noël, Angew. Chem. Int. Ed. 2018, 57, 4078–4082;
- 7cQ. Yang, Y.-H. Wang, Y. Qiao, M. Gau, P. J. Carroll, P. J. Walsh, E. J. Schelter, Science 2021, 372, 847–852;
- 7dM. I. Gonzalez, D. Gygi, Y. Qin, Q. Zhu, E. J. Johnson, Y.-S. Chen, D. G. Nocera, J. Am. Chem. Soc. 2022, 144, 1464–1472;
- 7eR. Lin, A. P. Amrute, J. Pérez-Ramírez, Chem. Rev. 2017, 117, 4182–4247.
- 8
- 8aL. Kesavan, R. Tiruvalam, M. H. A. Rahim, M. I. bin Saiman, D. I. Enache, R. L. Jenkins, N. Dimitratos, J. A. Lopez-Sanchez, S. H. Taylor, D. W. Knight, C. J. Kiely, G. J. Hutchings, Science 2011, 331, 195–199;
- 8bJ. A. B. Satrio, L. K. Doraiswamy, Chem. Eng. J. 2001, 82, 43–56.
- 9M. L. Alegre, M. Geronés, J. A. Rosso, S. G. Bertolotti, A. M. Braun, D. O. Mártire, M. C. Gonzalez, J. Phys. Chem. A 2000, 104, 3117–3125.
- 10Y. Lei, S. Cheng, N. Luo, X. Yang, T. An, Environ. Sci. Technol. 2019, 53, 11170–11182.
- 11G. G. Jayson, B. J. Parsons, A. J. Swallow, J. Chem. Soc. Faraday Trans. 1 1973, 69, 1597–1607.
- 12
- 12aW. Zhao, P. Yan, B. Li, M. Bahri, L. Liu, X. Zhou, R. Clowes, N. D. Browning, Y. Wu, J. W. Ward, A. I. Cooper, J. Am. Chem. Soc. 2022, 144, 9902–9909;
- 12bX. Yu, B. Viengkeo, Q. He, X. Zhao, Q. Huang, P. Li, W. Huang, Y. Li, Adv. Sustainable Syst. 2021, 5, 2100184;
- 12cC. Wu, Z. Teng, C. Yang, F. Chen, H. B. Yang, L. Wang, H. Xu, B. Liu, G. Zheng, Q. Han, Adv. Mater. 2022, 34, 2110266.
- 13
- 13aY. Shiraishi, T. Takii, T. Hagi, S. Mori, Y. Kofuji, Y. Kitagawa, S. Tanaka, S. Ichikawa, T. Hirai, Nat. Mater. 2019, 18, 985–993;
- 13bC. Yang, S. Wan, B. Zhu, J. Yu, S. Cao, Angew. Chem. Int. Ed. 2022, 61, e202208438.
- 14
- 14aS. Li, Z. Jin, X. Jiang, J. Yu, Y. Wang, S. Jin, H. Zhang, S. Song, T. Zeng, Chem. Eng. J. 2022, 431, 133900;
- 14bW. Huang, J. Byun, I. Rörich, C. Ramanan, P. W. M. Blom, H. Lu, D. Wang, L. Caire da Silva, R. Li, L. Wang, K. Landfester, K. A. I. Zhang, Angew. Chem. Int. Ed. 2018, 57, 8316–8320.
- 15
- 15aJ. Sun, H. Sekhar Jena, C. Krishnaraj, K. Singh Rawat, S. Abednatanzi, J. Chakraborty, A. Laemont, W. Liu, H. Chen, Y.-Y. Liu, K. Leus, H. Vrielinck, V. Van Speybroeck, P. Van Der Voort, Angew. Chem. Int. Ed. 2023, 62, e202216719;
- 15bM. Deng, J. Sun, A. Laemont, C. Liu, L. Wang, L. Bourda, J. Chakraborty, K. Van Hecke, R. Morent, N. De Geyter, K. Leus, H. Chen, P. Van Der Voort, Green Chem. 2023, 25, 3069–3076;
- 15cP. Das, G. Chakraborty, J. Roeser, S. Vogl, J. Rabeah, A. Thomas, J. Am. Chem. Soc. 2023, 145, 2975–2984;
- 15dY. Luo, B. Zhang, C. Liu, D. Xia, X. Ou, Y. Cai, Y. Zhou, J. Jiang, B. Han, Angew. Chem. Int. Ed. 2023, 62, e202305355.
- 16T. Sun, Y. Liang, W. Luo, L. Zhang, X. Cao, Y. Xu, Angew. Chem. Int. Ed. 2022, 61, e202203327.
- 17Z.-A. Lan, Y. Fang, Y. Zhang, X. Wang, Angew. Chem. Int. Ed. 2018, 57, 470–474.
- 18
- 18aB. Gu, W. Wu, G. Xu, G. Feng, F. Yin, P. H. J. Chong, J. Qu, K.-T. Yong, B. Liu, Adv. Mater. 2017, 29, 1701076;
- 18bK. Wen, H. Tan, Q. Peng, H. Chen, H. Ma, L. Wang, A. Peng, Q. Shi, X. Cai, H. Huang, Adv. Mater. 2022, 34, 2108146.
- 19F. Xiao, Z. Wang, J. Fan, T. Majima, H. Zhao, G. Zhao, Angew. Chem. Int. Ed. 2021, 60, 10375–10383.
- 20Y. Xing, Z. Yao, W. Li, W. Wu, X. Lu, J. Tian, Z. Li, H. Hu, M. Wu, Angew. Chem. Int. Ed. 2021, 60, 8889–8895.
- 21
- 21aC. Krishnaraj, H. Sekhar Jena, L. Bourda, A. Laemont, P. Pachfule, J. Roeser, C. V. Chandran, S. Borgmans, S. M. J. Rogge, K. Leus, C. V. Stevens, J. A. Martens, V. Van Speybroeck, E. Breynaert, A. Thomas, P. Van Der Voort, J. Am. Chem. Soc. 2020, 142, 20107–20116;
- 21bQ. Wu, J. Cao, X. Wang, Y. Liu, Y. Zhao, H. Wang, Y. Liu, H. Huang, F. Liao, M. Shao, Z. Kang, Nat. Commun. 2021, 12, 483.
- 22L. Chen, C. Chen, Z. Yang, S. Li, C. Chu, B. Chen, Adv. Funct. Mater. 2021, 31, 2105731.
- 23
- 23aN. Tsunoji, H. Nishida, Y. Ide, K. Komaguchi, S. Hayakawa, Y. Yagenji, M. Sadakane, T. Sano, ACS Catal. 2019, 9, 5742–5751;
- 23bK. Ohkubo, A. Fujimoto, S. Fukuzumi, Chem. Commun. 2011, 47, 8515–8517.
- 24L. Li, L. Xu, Z. Hu, J. C. Yu, Adv. Funct. Mater. 2021, 31, 2106120.
- 25
- 25aD. O. Mártire, J. A. Rosso, S. Bertolotti, G. C. Le Roux, A. M. Braun, M. C. Gonzalez, J. Phys. Chem. A 2001, 105, 5385–5392;
- 25bR. H. Schuler, G. Albarran, Radiat. Phys. Chem. 2002, 64, 189–195.