Engineered Histidine-Rich Peptides Enhance Endosomal Escape for Antibody-Targeted Intracellular Delivery of Functional Proteins
Graphical Abstract
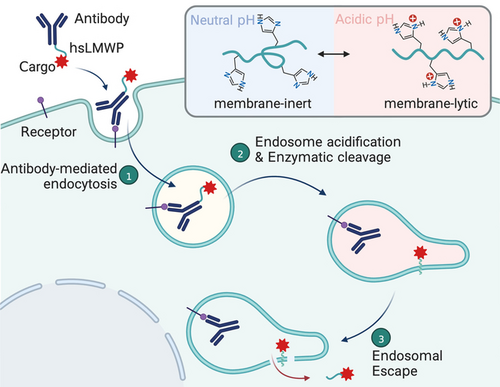
A new strategy has been developed to design and construct endosomal escape peptides. By substituting the Arg/Lys residues in cationic cell-penetrating peptides (CPPs) with histidine, peptides with pH-dependent membrane-perturbation activity have been obtained. These peptides can mediate the endosomal escape of peptide/protein cargoes upon antibody-targeted delivery, allowing potential applications such as protein delivery and gene editing.
Abstract
Currently, the clinical application of protein/peptide therapeutics is mainly limited to the modulation of diseases in extracellular spaces. Intracellular targets are hardly accessed, owing largely to the endosomal entrapment of internalized proteins/peptides. Here, we report a strategy to design and construct peptides that enable endosome-to-cytosol delivery based on an extension of the “histidine switch” principle. By substituting the Arg/Lys residues in cationic cell-penetrating peptides (CPPs) with histidine, we obtained peptides with pH-dependent membrane-perturbation activity. These peptides do not randomly penetrate cells like CPPs, but imitate the endosomal escape of CPPs following cellular uptake. Working with one such 16-residue peptide (hsLMWP) with high endosomal escape capacity, we engineered modular fusion proteins and achieved antibody-targeted delivery of diverse protein cargoes—including the pro-apoptotic protein BID (BH3-interacting domain death agonist) and Cre recombinase—into the cytosol of multiple cancer cell types. After extensive in vitro testing, an in vivo analysis with xenograft mice ultimately demonstrated that a trastuzumab-hsLMWP-BID fusion conferred strong anti-tumor efficacy without apparent side effects. Notably, our fusion protein features a modular design, allowing flexible applications for any antibody/cargo combination of choice. Therefore, the potential applications extend throughout life science and biomedicine, including gene editing, cancer treatment, and immunotherapy.
Conflict of interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.