Copper-Catalyzed Enantioselective C1,N-Dipolar (3+2) Cycloadditions of 2-Aminoallyl Cations with Indoles
Lulu Shen
State Key Laboratory and Institute of Elemento-Organic Chemistry, College of Chemistry, Nankai University, Tianjin, 300071 P. R. China
Search for more papers by this authorDr. Yin Zheng
State Key Laboratory of Synthetic Chemistry, Department of Chemistry, University of Hong Kong, Hong Kong, P. R. China
Search for more papers by this authorZitong Lin
State Key Laboratory and Institute of Elemento-Organic Chemistry, College of Chemistry, Nankai University, Tianjin, 300071 P. R. China
Search for more papers by this authorTianzhu Qin
State Key Laboratory and Institute of Elemento-Organic Chemistry, College of Chemistry, Nankai University, Tianjin, 300071 P. R. China
Search for more papers by this authorProf. Dr. Zhongxing Huang
State Key Laboratory of Synthetic Chemistry, Department of Chemistry, University of Hong Kong, Hong Kong, P. R. China
Search for more papers by this authorCorresponding Author
Prof. Dr. Weiwei Zi
State Key Laboratory and Institute of Elemento-Organic Chemistry, College of Chemistry, Nankai University, Tianjin, 300071 P. R. China
Haihe Laboratory of Sustainable Chemical Transformations, Tianjin, 300071 China
Search for more papers by this authorLulu Shen
State Key Laboratory and Institute of Elemento-Organic Chemistry, College of Chemistry, Nankai University, Tianjin, 300071 P. R. China
Search for more papers by this authorDr. Yin Zheng
State Key Laboratory of Synthetic Chemistry, Department of Chemistry, University of Hong Kong, Hong Kong, P. R. China
Search for more papers by this authorZitong Lin
State Key Laboratory and Institute of Elemento-Organic Chemistry, College of Chemistry, Nankai University, Tianjin, 300071 P. R. China
Search for more papers by this authorTianzhu Qin
State Key Laboratory and Institute of Elemento-Organic Chemistry, College of Chemistry, Nankai University, Tianjin, 300071 P. R. China
Search for more papers by this authorProf. Dr. Zhongxing Huang
State Key Laboratory of Synthetic Chemistry, Department of Chemistry, University of Hong Kong, Hong Kong, P. R. China
Search for more papers by this authorCorresponding Author
Prof. Dr. Weiwei Zi
State Key Laboratory and Institute of Elemento-Organic Chemistry, College of Chemistry, Nankai University, Tianjin, 300071 P. R. China
Haihe Laboratory of Sustainable Chemical Transformations, Tianjin, 300071 China
Search for more papers by this authorGraphical Abstract
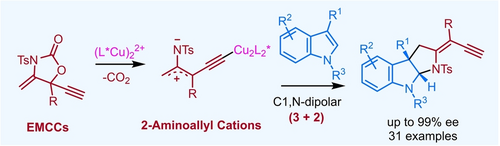
A binuclear copper-catalyzed (3+2) cycloaddition of 2-aminoallyl cations with indoles was achieved. This transformation featured a unique C1,N-dipolar cycloaddition reactivity for 2-aminoallyl cations. Enantioenriched pyrroloindoline derivatives were prepared by this cycloaddition in high yields with good enantio- and diastereoselectivities.
Abstract
2-Aminoallyl cations are versatile 1,3-dipoles that could potentially be used for diverse (3+n) cycloaddition reactions. Despite some preliminary studies, the asymmetric catalytic transformation of these species is still underdeveloped. We herein report a binuclear copper-catalyzed generation of 2-aminoallyl cations from ethynyl methylene cyclic carbamates and their enantioselective (3+2) cycloaddition reaction with indoles to construct chiral pyrroloindolines. This transformation features a novel C1,N-dipolar reactivity for 2-aminoallyl cations.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202217051-sup-0001-misc_information.pdf11.5 MB | Supporting Information |
| anie202217051-sup-0001-SI_checkcif.pdf164.6 KB | Supporting Information |
| anie202217051-sup-0001-SI_cu_1117_6_0m.cif1.1 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For reviews, see:
- 1aH. M. R. Hoffmann, Angew. Chem. Int. Ed. Engl. 1973, 12, 819–835; Angew. Chem. 1973, 85, 877–894;
- 1bR. Noyori, Y. Hayakawa, Org. React. 1983, 29, 163–344;
- 1cH. M. R. Hoffmann, Angew. Chem. Int. Ed. Engl. 1984, 23, 1–19; Angew. Chem. 1984, 96, 29–48;
- 1dJ. Mann, Tetrahedron 1986, 42, 4611–4659;
- 1eM. Harmata, Acc. Chem. Res. 2001, 34, 595–605;
- 1fM. Harmata, Chem. Commun. 2010, 46, 8886–8903;
- 1gM. Harmata, Chem. Commun. 2010, 46, 8904–8922;
- 1hA. G. Lohse, R. P. Hsung, Chem. Eur. J. 2011, 17, 3812–3822;
- 1iH. Li, J. Wu, Synthesis 2015, 47, 22–33.
- 2For non-enantioselective (4+3) cycloaddition reactions of oxyallyl cations, see:
- 2aA. W. Fort, J. Am. Chem. Soc. 1962, 84, 4979–4981;
- 2bH. Takaya, S. Makino, Y. Hayakawa, R. Noyori, J. Am. Chem. Soc. 1978, 100, 1765–1777;
- 2cR. Noyori, F. Shimizu, K. Fukuta, H. Takaya, Y. Hayakawa, J. Am. Chem. Soc. 1977, 99, 5196–5198;
- 2dM. Harmata, S. Elomari, C. L. Barnes, J. Am. Chem. Soc. 1996, 118, 2860–2871;
- 2eH. Xiong, R. P. Hsung, C. R. Berry, C. Rameshkumar, J. Am. Chem. Soc. 2001, 123, 7174–7175;
- 2fC. Rameshkumar, R. P. Hsung, Angew. Chem. Int. Ed. 2004, 43, 615–618; Angew. Chem. 2004, 116, 625–628;
- 2gW. K. Chung, S. K. Lam, B. Lo, L. L. Liu, W.-T. Wong, P. Chiu, J. Am. Chem. Soc. 2009, 131, 4556–4557;
- 2hJ. E. Antoline, E. H. Krenske, A. G. Lohse, K. N. Houk, R. P. Hsung, J. Am. Chem. Soc. 2011, 133, 14443–14451;
- 2iB. Lo, S. Lam, W.-T. Wong, P. Chiu, Angew. Chem. Int. Ed. 2012, 51, 12120–12123; Angew. Chem. 2012, 124, 12286–12289;
- 2jC. Fu, N. Lora, P. L. Kirchhoefer, D. R. Lee, E. Altenhofer, C. L. Barnes, N. L. Hungerford, E. H. Krenske, M. Harmata, Angew. Chem. Int. Ed. 2017, 56, 14682–14687; Angew. Chem. 2017, 129, 14874–14879.
- 3For non-enantioselective (3+2) cycloaddition reactions of oxyallyl cations, see:
- 3aH. Li, R. P. Hughes, J. Wu, J. Am. Chem. Soc. 2014, 136, 6288–6296;
- 3bK. Masuya, K. Domon, K. Tanino, I. Kuwajima, J. Am. Chem. Soc. 1998, 120, 1724–1731;
- 3cY. Chen, J. Ling, A. B. Keto, Y. He, K.-H. Low, E. H. Krenske, P. Chiu, Angew. Chem. Int. Ed. 2022, 61, e202116099; Angew. Chem. 2022, 134, e202116099.
- 4For enantioselective (4+3) cycloaddition reactions of oxyallyl cations with furans, see:
- 4aM. Harmata, S. K. Ghosh, X. Hong, S. Wacharasindhu, P. Kirchhoefer, J. Am. Chem. Soc. 2003, 125, 2058–2059;
- 4bJ. Huang, R. P. Hsung, J. Am. Chem. Soc. 2005, 127, 50–51;
- 4cM. Topinka, K. Zawatzky, C. L. Barnes, C. J. Welch, M. Harmata, Org. Lett. 2017, 19, 4106–4109;
- 4dL. Villar, U. Uria, J. I. Martinez, L. Prieto, E. Reyes, L. Carrillo, J. L. Vicario, Angew. Chem. Int. Ed. 2017, 56, 10535–10538; Angew. Chem. 2017, 129, 10671–10674;
- 4eS. M. Banik, A. Levina, A. M. Hyde, E. N. Jacobsen, Science 2017, 358, 761–764.
- 5
- 5aB. M. Trost, Z. Huang, G. M. Murhade, Science 2018, 362, 564–568;
- 5bY. Zou, S. Chen, K. N. Houk, J. Am. Chem. Soc. 2019, 141, 12382–12387;
- 5cB. M. Trost, Z. Huang, Angew. Chem. Int. Ed. 2019, 58, 6396–6399; Angew. Chem. 2019, 131, 6462–6465.
- 6W. Chai, Q. Zhou, W. Ai, Y. Zheng, T. Qin, X. Xu, W. Zi, J. Am. Chem. Soc. 2021, 143, 3595–3603.
- 7Y. Zheng, T. Qin, W. Zi, J. Am. Chem. Soc. 2021, 143, 1038–1045.
- 8W. Zhang, P.-C. Zhang, Y.-L. Li, H.-H. Wu, J. Zhang, J. Am. Chem. Soc. 2022, 144, 19627–19634.
- 9G. Prié, N. Prévost, H. Twin, S. A. Fernandes, J. F. Hayes, M. Shipman, Angew. Chem. Int. Ed. 2004, 43, 6517–6519; Angew. Chem. 2004, 116, 6679–6681.
- 10For preliminary studies on 2-aminoallyl cations, see
- 10aR. Schmid, H. Schmid, Helv. Chim. Acta 1974, 57, 1883–1886;
- 10bJ. Lee, J. Oh, S.-J. Jin, J.-R. Choi, J. L. Atwood, J. K. Cha, J. Org. Chem. 1994, 59, 6955–6964;
- 10cH. Kim, C. Ziani-Cherif, J. Oh, D. Lee, J. K. Cha, J. Org. Chem. 1995, 60, 792–793;
- 10dA. S. Kende, H. Huang, Tetrahedron Lett. 1997, 38, 3353–3356.
- 11L. Shen, Z. Lin, B. Guo, W. Zi, Nat. Synth. 2022, 1, 883–891.
- 12
- 12aG.-J. Mei, W. L. Koay, C. X. A. Tan, Y. Lu, Chem. Soc. Rev. 2021, 50, 5985–6012;
- 12bL. M. Repka, S. E. Reisman, J. Org. Chem. 2013, 78, 12314–12320.
- 13
- 13aY.-N. Wang, T.-R. Li, M.-M. Zhang, B.-Y. Cheng, L.-Q. Lu, W.-J. Xiao, J. Org. Chem. 2016, 81, 10491–10498;
- 13bE. Rossi, G. Abbiati, M. Dell'Acqua, M. Negrato, A. Paganoni, V. Pirovano, Org. Biomol. Chem. 2016, 14, 6095–6110;
- 13cA. Mal, M. Sayyad, I. A. Wani, M. K. Ghorai, J. Org. Chem. 2017, 82, 4–11;
- 13dZ. Chai, Y.-M. Zhu, P.-J. Yang, S. Wang, S. Wang, Z. Liu, G. Yang, J. Am. Chem. Soc. 2015, 137, 10088–10091.
- 14
- 14aM. C. DiPoto, R. P. Hughes, J. Wu, J. Am. Chem. Soc. 2015, 137, 14861–14864;
- 14bW. Ji, L. Yao, X. Liao, Org. Lett. 2016, 18, 628–630.
- 15Deposition number 2083423 (3 ae) contains the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service.
- 16See the Supporting Information for mechanistic consideration of this rearrangement. We thank the reviewers for their suggestions, which resulted in the discovery of this rearrangement.
- 17For examples of dinuclear copper catalyst, see:
- 17aR.-Z. Li, D.-Q. Liu, D. Niu, Nat. Catal. 2020, 3, 672–680;
- 17bK. Nakajima, M. Shibata, Y. Nishibayashi, J. Am. Chem. Soc. 2015, 137, 2472–2475.
- 18For reviews on Cu-catalyzed propargylic decarboxylation substitution:
- 18aY. Miyake, S. Uemura, Y. Nishibayashi, ChemCatChem 2009, 1, 342–356;
- 18bR. Roy, S. Saha, RSC Adv. 2018, 8, 31129–31193;
- 18cD.-Y. Zhang, X.-P. Hu, Tetrahedron Lett. 2015, 56, 283–295.
- 19For selected reports on Cu-catalyzed propargylic decarboxylation substitution:
- 19aF.-L. Zhu, Y. Zou, D.-Y. Zhang, Y.-H. Wang, X.-H. Hu, S. Chen, J. Xu, X.-P. Hu, Angew. Chem. Int. Ed. 2014, 53, 1410–1414; Angew. Chem. 2014, 126, 1434–1438;
- 19bK. Zhang, L.-Q. Lu, S. Yao, J.-R. Chen, D.-Q. Shi, W.-J. Xiao, J. Am. Chem. Soc. 2017, 139, 12847–12854;
- 19cK. Tsuchida, Y. Senda, K. Nakajima, Y. Nishibayashi, Angew. Chem. Int. Ed. 2016, 55, 9728–9732; Angew. Chem. 2016, 128, 9880–9884;
- 19dR.-Z. Li, H. Tang, K. R. Yang, L.-Q. Wan, X. Zhang, J. Liu, Z. Fu, D. Niu, Angew. Chem. Int. Ed. 2017, 56, 7213–7217; Angew. Chem. 2017, 129, 7319–7323;
- 19eJ. E. Gómez, W. Guo, S. Gaspa, A. W. Kleij, Angew. Chem. Int. Ed. 2017, 56, 15035–15038; Angew. Chem. 2017, 129, 15231–15234;
- 19fJ. E. Gómez, À. Cristòfol, A. W. Kleij, Angew. Chem. Int. Ed. 2019, 58, 3903–3907; Angew. Chem. 2019, 131, 3943–3947;
- 19gW. Guo, L. Zuo, M. Cui, B. Yan, S. Ni, J. Am. Chem. Soc. 2021, 143, 7629–7634.
- 20C. Liu, E. Z. Oblak, M. N. V. Wal, A. K. Dilger, D. K. Almstead, D. W. C. MacMillan, J. Am. Chem. Soc. 2016, 138, 2134–2137.
- 21E. H. Krenske, S. He, J. Huang, Y. Du, K. N. Houk, R. P. Hsung, J. Am. Chem. Soc. 2013, 135, 5242–5245.
- 22For a review on copper-catalyzed cycloadditions involving propargylic carboxylates, see: X.-H. Hu, Z.-T. Liu, L. Shao, X.-P. Hu, Synthesis 2015, 47, 913–923. Selected examples:
- 22aC. Zhang, X.-H. Hu, Y.-H. Wang, Z. Zheng, J. Xu, X.-P. Hu, J. Am. Chem. Soc. 2012, 134, 9585–9588;
- 22bF.-L. Zhu, Y.-H. Wang, D.-Y. Zhang, J. Xu, X.-P. Hu, Angew. Chem. Int. Ed. 2014, 53, 10223–10227; Angew. Chem. 2014, 126, 10387–10391;
- 22cD.-Y. Zhang, L. Shao, J. Xu, X.-P. Hu, ACS Catal. 2015, 5, 5026–5030;
- 22dL. Shao, Y.-H. Wang, D.-Y. Zhang, J. Xu, X.-P. Hu, Angew. Chem. Int. Ed. 2016, 55, 5014–5018; Angew. Chem. 2016, 128, 5098–5102;
- 22eQ. Wang, T.-R. Li, L.-Q. Lu, M.-M. Li, K. Zhang, W.-J. Xiao, J. Am. Chem. Soc. 2016, 138, 8360–8363;
- 22fJ. Song, Z.-J. Zhang, L.-Z. Gong, Angew. Chem. Int. Ed. 2018, 57, 3269–3281; Angew. Chem. 2018, 130, 3325–3337.