Bacterial Avenalumic Acid Biosynthesis Includes Substitution of an Aromatic Amino Group for Hydride by Nitrous Acid Dependent Diazotization
Seiji Kawai
Department of Biotechnology, Graduate School of Agricultural and Life Sciences, The University of Tokyo, 1-1-1 Yayoi, Bunkyo-ku, Tokyo, 113-8657 Japan
Search for more papers by this authorRyota Hagihara
Department of Biotechnology, Graduate School of Agricultural and Life Sciences, The University of Tokyo, 1-1-1 Yayoi, Bunkyo-ku, Tokyo, 113-8657 Japan
Search for more papers by this authorProf. Dr. Kazuo Shin-ya
National Institute of Advanced Industrial Science and Technology (AIST), 2-4-7 Aomi, Koto-ku, Tokyo, 135-0064 Japan
Biotechnology Research Center, The University of Tokyo, 1-1-1 Yayoi, Bunkyo-ku, Tokyo, 113-8657 Japan
Search for more papers by this authorCorresponding Author
Prof. Dr. Yohei Katsuyama
Department of Biotechnology, Graduate School of Agricultural and Life Sciences, The University of Tokyo, 1-1-1 Yayoi, Bunkyo-ku, Tokyo, 113-8657 Japan
Collaborative Research Institute for Innovative Microbiology, The University of Tokyo Bunkyo-ku, Tokyo, 113-8657 Japan
Search for more papers by this authorProf. Dr. Yasuo Ohnishi
Department of Biotechnology, Graduate School of Agricultural and Life Sciences, The University of Tokyo, 1-1-1 Yayoi, Bunkyo-ku, Tokyo, 113-8657 Japan
Collaborative Research Institute for Innovative Microbiology, The University of Tokyo Bunkyo-ku, Tokyo, 113-8657 Japan
Search for more papers by this authorSeiji Kawai
Department of Biotechnology, Graduate School of Agricultural and Life Sciences, The University of Tokyo, 1-1-1 Yayoi, Bunkyo-ku, Tokyo, 113-8657 Japan
Search for more papers by this authorRyota Hagihara
Department of Biotechnology, Graduate School of Agricultural and Life Sciences, The University of Tokyo, 1-1-1 Yayoi, Bunkyo-ku, Tokyo, 113-8657 Japan
Search for more papers by this authorProf. Dr. Kazuo Shin-ya
National Institute of Advanced Industrial Science and Technology (AIST), 2-4-7 Aomi, Koto-ku, Tokyo, 135-0064 Japan
Biotechnology Research Center, The University of Tokyo, 1-1-1 Yayoi, Bunkyo-ku, Tokyo, 113-8657 Japan
Search for more papers by this authorCorresponding Author
Prof. Dr. Yohei Katsuyama
Department of Biotechnology, Graduate School of Agricultural and Life Sciences, The University of Tokyo, 1-1-1 Yayoi, Bunkyo-ku, Tokyo, 113-8657 Japan
Collaborative Research Institute for Innovative Microbiology, The University of Tokyo Bunkyo-ku, Tokyo, 113-8657 Japan
Search for more papers by this authorProf. Dr. Yasuo Ohnishi
Department of Biotechnology, Graduate School of Agricultural and Life Sciences, The University of Tokyo, 1-1-1 Yayoi, Bunkyo-ku, Tokyo, 113-8657 Japan
Collaborative Research Institute for Innovative Microbiology, The University of Tokyo Bunkyo-ku, Tokyo, 113-8657 Japan
Search for more papers by this authorGraphical Abstract
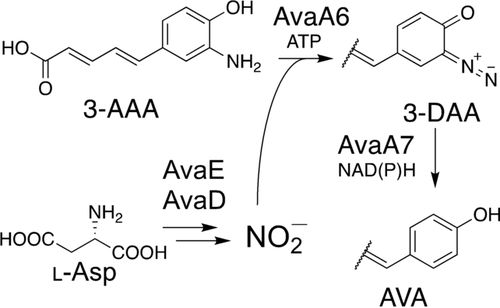
The diazo group is highly reactive and provides biological activity to natural products. By genome mining of the diazo group-biosynthesizing enzymes, an avenalumic acid (AVA) biosynthetic gene cluster was identified in Streptomyces sp. RI-77. This pathway includes two enzymes, AvaA6 and AvaA7, catalyzing the diazotization of 3-aminoavenalumic acid using nitrous acid and substitution of the diazo group for hydride to synthesize AVA, respectively.
Abstract
The diazo group is an important functional group that can confer biological activity to natural products owing to its high reactivity. Recent studies have revealed that diazo groups are synthesized from amino groups using nitrous acid in secondary metabolites of actinomycetes. However, genome database analysis indicated that there are still many diazo group-biosynthesizing enzymes for unknown biosynthetic pathways. Here, we discovered an avenalumic acid biosynthesis gene cluster in Streptomyces sp. RI-77 by genome mining of enzymes involved in diazo group formation. Through heterologous expression, the gene cluster was revealed to direct avenalumic acid (AVA) biosynthesis via 3-aminoavenalumic acid (3-AAA). In vitro enzyme assays showed that AvaA6 and AvaA7 catalyzed the diazotization of 3-AAA using nitrous acid and substitution of the diazo group for hydride to synthesize AVA, respectively. This study revealed an unprecedented pathway for amino group removal via diazotization.
Conflict of interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202211728-sup-0001-misc_information.pdf7.3 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1L. Katz, R. H. Baltz, J. Ind. Microbiol. Biotechnol. 2016, 43, 155–176.
- 2G. Le Goff, J. Ouazzani, Bioorg. Med. Chem. 2014, 22, 6529–6544.
- 3C. C. Nawrat, C. J. Moody, Nat. Prod. Rep. 2011, 28, 1426–1444.
- 4L. M. Blair, J. Sperry, J. Nat. Prod. 2013, 76, 794–812.
- 5Y. Katsuyama, K. Matsuda, Curr. Opin. Chem. Biol. 2020, 59, 62–68.
- 6L. Chen, Z. Deng, C. Zhao, ACS Chem. Biol. 2021, 16, 559–570.
- 7H.-Y. He, H. Niikura, Y.-L. Du, K. S. Ryan, Chem. Soc. Rev. 2022, 51, 2991–3046.
- 8T. Ye, M. A. McKervey, Chem. Rev. 1994, 94, 1091–1160.
- 9A. Ford, H. Miel, A. Ring, C. N. Slattery, A. R. Maguire, M. A. McKervey, Chem. Rev. 2015, 115, 9981–10080.
- 10L. C. Colis, C. M. Woo, D. C. Hegan, Z. Li, P. M. Glazer, S. B. Herzon, Nat. Chem. 2014, 6, 504–510.
- 11K. A. Mix, M. R. Aronoff, R. T. Raines, ACS Chem. Biol. 2016, 11, 3233–3244.
- 12Y. Sugai, Y. Katsuyama, Y. Ohnishi, Nat. Chem. Biol. 2016, 12, 73–75.
- 13Y. Katsuyama, Y. Sato, Y. Sugai, Y. Higashiyama, M. Senda, T. Senda, Y. Ohnishi, FEBS J. 2018, 285, 1540–1555.
- 14K.-K. A. Wang, T. L. Ng, P. Wang, Z. Huang, E. P. Balskus, W. A. van der Donk, Nat. Commun. 2018, 9, 3687.
- 15T. L. Ng, M. E. McCallum, C. R. Zheng, J. X. Wang, K. J. Y. Wu, E. P. Balskus, ChemBioChem 2020, 21, 1155–1160.
- 16S. Kawai, Y. Sugaya, R. Hagihara, H. Tomita, Y. Katsuyama, Y. Ohnishi, Angew. Chem. Int. Ed. 2021, 60, 10319–10325; Angew. Chem. 2021, 133, 10407–10413.
- 17A. Del Rio Flores, F. F. Twigg, Y. Du, W. Cai, D. Q. Aguirre, M. Sato, M. J. Dror, M. Narayanamoorthy, J. Geng, N. A. Zill, R. Zhai, W. Zhang, Nat. Chem. Biol. 2021, 17, 1305–1313.
- 18G.-L. Ma, H. Candra, L. M. Pang, J. Xiong, Y. Ding, H. T. Tran, Z. J. Low, H. Ye, M. Liu, J. Zheng, M. Fang, B. Cao, Z.-X. Liang, J. Am. Chem. Soc. 2022, 144, 1622–1633.
- 19M. Wang, H. Niikura, H.-Y. He, P. Daniel-Ivad, K. S. Ryan, Angew. Chem. Int. Ed. 2020, 59, 3881–3885; Angew. Chem. 2020, 132, 3909–3913.
- 20A. J. Waldman, E. P. Balskus, J. Org. Chem. 2018, 83, 7539–7546.
- 21F. F. Twigg, W. Cai, W. Huang, J. Liu, M. Sato, T. J. Perez, J. Geng, M. J. Dror, I. Montanez, T. L. Tong, H. Lee, W. Zhang, ChemBioChem 2019, 20, 1145–1149.
- 22R. Hagihara, Y. Katsuyama, Y. Sugai, H. Onaka, Y. Ohnishi, J. Antibiot. 2018, 71, 911–919.
- 23D. P. Labeda, J. R. Doroghazi, K.-S. Ju, W. W. Y. Metcalf, Int. J. Systematic Evolutionary Microbiol. 2014, 64, 894–900.
- 24M. Izumikawa, R. Satou, K. Motohashi, A. Nagai, Y. Ohnishi, M. Takagi, K. Shin-ya, J. Nat. Prod. 2011, 74, 2588–2591.
- 25K. Motohashi, M. Izumikawa, N. Kagaya, M. Takagi, K. Shin-ya, J. Antibiot. 2016, 69, 707–708.
- 26Y. Katsuyama, K. Sone, R. Satou, M. Izumikawa, M. Takagi, M. Fujie, N. Satoh, K. Shin-ya, Y. Ohnishi, ChemBioChem 2016, 17, 1021–1028.
- 27Y.-Y. Guo, H. Li, Z.-X. Zhou, X.-M. Mao, Y. Tang, X. Chen, X.-H. Jiang, Y. Liu, H. Jiang, Y.-Q. Li, Org. Lett. 2015, 17, 6114–6117.
- 28D. Du, Y. Katsuyama, K. Shin-ya, Y. Ohnishi, Angew. Chem. Int. Ed. 2018, 57, 1954–1957; Angew. Chem. 2018, 130, 1972–1975.
- 29S. Pohle, C. Appelt, M. Roux, H.-P. Fiedler, R. D. Süssmuth, J. Am. Chem. Soc. 2011, 133, 6194–6205.
- 30Z. Rui, K. Petříčková, F. Škanta, S. Pospíšil, Y. Yang, C.-Y. Chen, S.-F. Tsai, H. G. Floss, M. Petříček, T.-W. Yu, J. Biol. Chem. 2010, 285, 24915–24924.
- 31J. Zhang, S. Yuzawa, W. L. Thong, T. Shinada, M. Nishiyama, T. Kuzuyama, J. Am. Chem. Soc. 2021, 143, 2962–2969.
- 32G. L. C. Grammbitter, M. Schmalhofer, K. Karimi, Y.-M. Shi, T. A. Schöner, N. J. Tobias, N. Morgner, M. Groll, H. B. Bode, J. Am. Chem. Soc. 2019, 141, 16615–16623.
- 33K. Petříčková, S. Pospíšil, M. Kuzma, T. Tylová, M. Jágr, P. Tomek, A. Chroňáková, E. Brabcová, L. Anděra, V. Krištůfek, M. Petříček, ChemBioChem 2014, 15, 1334–1345.
- 34O. Bilyk, E. Brötz, B. Tokovenko, A. Bechthold, T. Paululat, A. Luzhetskyy, ACS Chem. Biol. 2016, 11, 241–250.
- 35P. Cimermancic, M. H. Medema, J. Claesen, K. Kurita, L. C. Wieland Brown, K. Mavrommatis, A. Pati, P. A. Godfrey, M. Koehrsen, J. Clardy, B. W. Birren, E. Takano, A. Sali, R. G. Linington, M. A. Fischbach, Cell 2014, 158, 412–421.
- 36F. W. Collins, D. C. McLachlan, B. A. Blackwell, Cereal Chem. 1991, 68, 184–189.
- 37S. Kawai, Y. Katsuyama, Y. Ohnishi, ChemBioChem 2022, 23, e202100700.
- 38J. Mistry, S. Chuguransky, L. Williams, M. Qureshi, G. A. Salazar, E. L. L. Sonnhammer, S. C. E. Tosatto, L. Paladin, S. Raj, L. J. Richardson, R. D. Finn, A. Bateman, Nucleic Acids Res. 2021, 49, D412–D419.
- 39E. R. Hoffarth, K. W. Rothchild, K. S. Ryan, FEBS J. 2020, 287, 1403–1428.
- 40I. Johnston, L. J. Osborn, R. L. Markley, E. A. McManus, A. Kadam, K. B. Schultz, N. Nagajothi, P. P. Ahern, J. M. Brown, J. Claesen, npj Biofilms Microbioms 2021, 7, 56.
- 41R. Meganathan, FEMS Microbiol. Lett. 2001, 203, 131–139.
- 42M. Kanehisa, S. Goto, S. Kawashima, A. Nakaya, Nucleic Acids Res. 2002, 30, 42–46.
- 43R. G. Sawers, D. Falke, M. Fischer, in Advances in Microbial Physiology (Ed.: R. K. Poole), Academic Press, San Diego, 2016, pp. 1–40.
10.1016/bs.ampbs.2016.07.003 Google Scholar
- 44Y. Yang, L. Fu, J. Zhang, L. Hu, M. Xu, J. Xu, PLoS One 2014, 9, e99537.
- 45S. A. Kautsar, K. Blin, S. Shaw, J. C. Navarro-Muñoz, B. R. Terlouw, J. J. J. van der Hooft, J. A. van Santen, V. Tracanna, H. G. Suarez Duran, V. Pascal Andreu, N. Selem-Mojica, M. Alanjary, S. L. Robinson, G. Lund, S. C. Epstein, A. C. Sisto, L. K. Charkoudian, J. Collemare, R. G. Linington, T. Weber, M. H. Medema, Nucleic Acids Res. 2019, gkz882.
- 46H. Suzuki, Y. Ohnishi, Y. Furusho, S. Sakuda, S. Horinouchi, J. Biol. Chem. 2006, 281, 36944–36951.
- 47Y. Dashti, T. Grkovic, U. R. Abdelmohsen, U. Hentschel, R. J. Quinn, J. Nat. Prod. 2017, 80, 828–836.
- 48A. Ishihara, Y. Ohtsu, H. Iwamura, Phytochemistry 1999, 50, 237–242.
- 49A. Ishihara, Y. Ohtsu, H. Iwamura, Planta 1999, 208, 512–518.