Aggregation-Dependent Circularly Polarized Luminescence and Thermally Activated Delayed Fluorescence from Chiral Carbene-CuI-Amide Enantiomers
Graphical Abstract
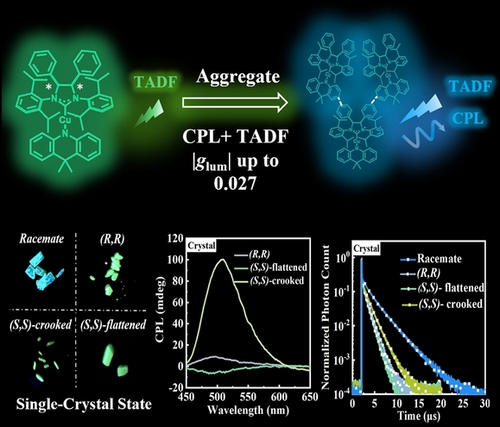
A pair of chiral CuI-based CMA enantiomers, (R,R)-PSIPr*-Cu-DMAC and (S,S)-PSIPr*-Cu-DMAC, have been constructed to exhibit aggregation-dependent circularly polarized luminescence with large luminescence dissymmetry factor of up to +0.027. Inspiring aggregation-dependent TADF properties strongly associated with ligand-ligand rotation of enantiomer molecules were also demonstrated.
Abstract
The field of luminescent carbene-metal-amide (CMA) complexes and chiroptical-active materials has been blossoming in recent years, although chiroptical-active CMA complexes have not been reported so far. For the first time, a pair of chiral CuI-based CMA enantiomers, (R,R)-PSIPr*-Cu-DMAC and (S,S)-PSIPr*-Cu-DMAC, have been developed by using chiral phenyl-substituted N-heterocyclic carbenes as acceptor ligands in the CMA motif. The CuI-based CMA enantiomers exhibited aggregation-induced circularly polarized luminescence with a large luminescence dissymmetry factor of up to +0.027, the first reported for CMA complexes. This success originates from the limited ligand-ligand rotation freedom and asymmetrical packing pattern (helical structure) of the CMA enantiomers in the crystals. Moreover, these CuI enantiomers displayed inspiring aggregation-dependent thermally activated delayed fluorescence properties. These findings bring new insights into the optical properties of chiral CMA complexes from the perspective of aggregation states.
Conflict of interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.