Affinity Bioorthogonal Chemistry (ABC) Tags for Site-Selective Conjugation, On-Resin Protein-Protein Coupling, and Purification of Protein Conjugates
Corresponding Author
Samuel L. Scinto
Department of Chemistry and Biochemistry, University of Delaware, Ammon Pinizzotto Biopharmaceutical Innovation Center, Newark, DE 19713 USA
Search for more papers by this authorTyler R. Reagle
Department of Chemistry and Biochemistry, University of Delaware, Ammon Pinizzotto Biopharmaceutical Innovation Center, Newark, DE 19713 USA
Search for more papers by this authorCorresponding Author
Joseph M. Fox
Department of Chemistry and Biochemistry, University of Delaware, Ammon Pinizzotto Biopharmaceutical Innovation Center, Newark, DE 19713 USA
Search for more papers by this authorCorresponding Author
Samuel L. Scinto
Department of Chemistry and Biochemistry, University of Delaware, Ammon Pinizzotto Biopharmaceutical Innovation Center, Newark, DE 19713 USA
Search for more papers by this authorTyler R. Reagle
Department of Chemistry and Biochemistry, University of Delaware, Ammon Pinizzotto Biopharmaceutical Innovation Center, Newark, DE 19713 USA
Search for more papers by this authorCorresponding Author
Joseph M. Fox
Department of Chemistry and Biochemistry, University of Delaware, Ammon Pinizzotto Biopharmaceutical Innovation Center, Newark, DE 19713 USA
Search for more papers by this authorGraphical Abstract
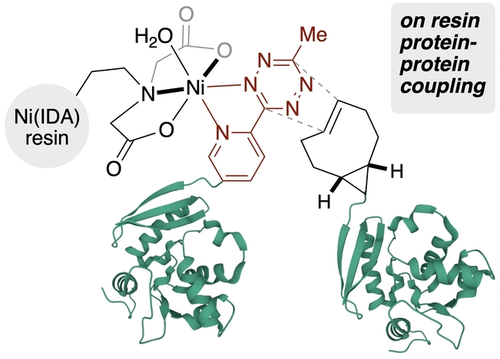
Affinity Bioorthogonal Chemistry tags (ABC-tags) based on 2-pyridyltetrazines serve a dual role by enabling protein purification through metal chelation and still maintaining their rapid kinetics in bioorthogonal reactions. ABC-tagging is successful for proteins tagged at the C- or N-terminus or at internal positions, is successful in complex mixtures including cell lysate, and can be carried out on-resin to generate multidomain proteins.
Abstract
The site-selective functionalization of proteins has broad application in chemical biology, but can be limited when mixtures result from incomplete conversion or the formation of protein containing side products. It is shown here that when proteins are covalently tagged with pyridyl-tetrazines, the nickel-iminodiacetate (Ni-IDA) resins commonly used for His-tags can be directly used for protein affinity purification. These Affinity Bioorthogonal Chemistry (ABC) tags serve a dual role by enabling affinity-based protein purification while maintaining rapid kinetics in bioorthogonal reactions. ABC-tagging works with a range of site-selective bioconjugation methods with proteins tagged at the C-terminus, N-terminus or at internal positions. ABC-tagged proteins can also be purified from complex mixtures including cell lysate. The combination of site-selective conjugation and clean-up with ABC-tagged proteins also allows for facile on-resin reactions to provide protein-protein conjugates.
Conflict of interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202207661-sup-0001-misc_information.pdf13.2 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1A. F. L. Schneider, C. P. R. Hackenberger, Curr. Opin. Biotechnol. 2017, 48, 61–68.
- 2C. G. Parker, M. R. Pratt, Cell 2020, 180, 605–632.
- 3P. Agarwal, C. R. Bertozzi, Bioconjugate Chem. 2015, 26, 176–192.
- 4C. D. Spicer, E. T. Pashuck, M. M. Stevens, Chem. Rev. 2018, 118, 7702–7743.
- 5S. L. Scinto, D. A. Bilodeau, R. Hincapie, W. Lee, S. S. Nguyen, M. Xu, C. W. am Ende, M. G. Finn, K. Lang, Q. Lin, J. P. Pezacki, J. A. Prescher, M. S. Robillard, J. M. Fox, Nat. Rev. Methods Primers 2021, 1, 30.
- 6C. D. Spicer, B. G. Davis, Nat. Commun. 2014, 5, 4740.
- 7
- 7aC. B. Rosen, M. B. Francis, Nat. Chem. Biol. 2017, 13, 697–705;
- 7bP. E. Dawson, T. W. Muir, I. Clark-Lewis, S. B. Kent, Science 1994, 266, 776–779;
- 7cJ. B. Blanco-Canosa, P. E. Dawson, Angew. Chem. Int. Ed. 2008, 47, 6851–6855; Angew. Chem. 2008, 120, 6957–6961;
- 7dT. W. Muir, D. Sondhi, P. A. Cole, Proc. Natl. Acad. Sci. USA 1998, 95, 6705–6710;
- 7eN. J. Mitchell, L. R. Malins, X. Liu, R. E. Thompson, B. Chan, L. Radom, R. J. Payne, J. Am. Chem. Soc. 2015, 137, 14011–14014;
- 7fH. Ren, F. Xiao, K. Zhan, Y. P. Kim, H. Xie, Z. Xia, J. Rao, Angew. Chem. Int. Ed. 2009, 48, 9658–9662; Angew. Chem. 2009, 121, 9838–9842;
- 7gJ. I. MacDonald, H. K. Munch, T. Moore, M. B. Francis, Nat. Chem. Biol. 2015, 11, 326–331.
- 8
- 8aC. S. Theile, M. D. Witte, A. E. Blom, L. Kundrat, H. L. Ploegh, C. P. Guimaraes, Nat. Protoc. 2013, 8, 1800–1807;
- 8bA. M. Weeks, J. A. Wells, Nat. Chem. Biol. 2018, 14, 50–57;
- 8cG. K. Nguyen, Y. Cao, W. Wang, C. F. Liu, J. P. Tam, Angew. Chem. Int. Ed. 2015, 54, 15694–15698; Angew. Chem. 2015, 127, 15920–15924;
- 8dC. Kulkarni, T. L. Kinzer-Ursem, D. A. Tirrell, ChemBioChem 2013, 14, 1958–1962;
- 8eL. S. Witus, T. Moore, B. W. Thuronyi, A. P. Esser-Kahn, R. A. Scheck, A. T. Iavarone, M. B. Francis, J. Am. Chem. Soc. 2010, 132, 16812–16817.
- 9
- 9aD. Schumacher, J. Helma, F. A. Mann, G. Pichler, F. Natale, E. Krause, M. C. Cardoso, C. P. Hackenberger, H. Leonhardt, Angew. Chem. Int. Ed. 2015, 54, 13787–13791; Angew. Chem. 2015, 127, 13992–13996;
- 9bC. P. Guimaraes, M. D. Witte, C. S. Theile, G. Bozkurt, L. Kundrat, A. E. Blom, H. L. Ploegh, Nat. Protoc. 2013, 8, 1787–1799;
- 9cF. B. H. Rehm, T. J. Harmand, K. Yap, T. Durek, D. J. Craik, H. L. Ploegh, J. Am. Chem. Soc. 2019, 141, 17388–17393.
- 10
- 10aM. Fernández-Suárez, H. Baruah, L. Martinez-Hernandez, K. T. Xie, J. M. Baskin, C. R. Bertozzi, A. Y. Ting, Nat. Biotechnol. 2007, 25, 1483–1487;
- 10bE. de Boer, P. Rodriguez, E. Bonte, J. Krijgsveld, E. Katsantoni, A. Heck, F. Grosveld, J. Strouboulis, Proc. Natl. Acad. Sci. USA 2003, 100, 7480–7485;
- 10cA. Lopez Aguilar, J. G. Briard, L. Yang, B. Ovryn, M. S. Macauley, P. Wu, ACS Chem. Biol. 2017, 12, 611–621.
- 11C. L. Young, Z. T. Britton, A. S. Robinson, Biotechnol. J. 2012, 7, 620–634.
- 12R. L. Policarpo, H. Kang, X. Liao, A. E. Rabideau, M. D. Simon, B. L. Pentelute, Angew. Chem. Int. Ed. 2014, 53, 9203–9208; Angew. Chem. 2014, 126, 9357–9362.
- 13J. E. Cronan Jr., J. Biol. Chem. 1990, 265, 10327–10333.
- 14
- 14aC. B. Rosen, R. L. Kwant, J. I. MacDonald, M. Rao, M. B. Francis, Angew. Chem. Int. Ed. 2016, 55, 8585–8589; Angew. Chem. 2016, 128, 8727–8731;
- 14bT. Nguyen, N. S. Joshi, M. B. Francis, Bioconjugate Chem. 2006, 17, 869–872.
- 15M. M. Zegota, T. Wang, C. Seidler, D. Y. Wah Ng, S. L. Kuan, T. Weil, Bioconjugate Chem. 2018, 29, 2665–2670.
- 16
- 16aM. A. Nessen, G. Kramer, J. Back, J. M. Baskin, L. E. Smeenk, L. J. de Koning, J. H. van Maarseveen, L. de Jong, C. R. Bertozzi, H. Hiemstra, C. G. de Koster, J. Proteome Res. 2009, 8, 3702–3711;
- 16bS. Biedka, B. F. Schmidt, N. M. Frey, S. M. Boothman, J. S. Minden, A. L. Wilson, J. Proteome Res. 2021, 20, 4787–4800.
- 17
- 17aM. L. Blackman, M. Royzen, J. M. Fox, J. Am. Chem. Soc. 2008, 130, 13518–13519;
- 17bM. T. Taylor, M. L. Blackman, O. Dmitrenko, J. M. Fox, J. Am. Chem. Soc. 2011, 133, 9646–9649;
- 17cA. Darko, S. Wallace, O. Dmitrenko, M. M. Machovina, R. A. Mehl, J. W. Chin, J. M. Fox, Chem. Sci. 2014, 5, 3770–3776;
- 17dW. D. Lambert, S. L. Scinto, O. Dmitrenko, S. J. Boyd, R. Magboo, R. A. Mehl, J. W. Chin, J. M. Fox, S. Wallace, Org. Biomol. Chem. 2017, 15, 6640–6644.
- 18
- 18aW. D. Lambert, Y. Fang, S. Mahapatra, Z. Huang, C. W. am Ende, J. M. Fox, J. Am. Chem. Soc. 2019, 141, 17068–17074;
- 18bY. Xie, Y. Fang, Z. Huang, A. M. Tallon, C. W. am Ende, J. M. Fox, Angew. Chem. Int. Ed. 2020, 59, 16967–16973; Angew. Chem. 2020, 132, 17115–17121.
- 19
- 19aH. E. Murrey, J. C. Judkins, C. W. am Ende, T. E. Ballard, Y. Fang, K. Riccardi, L. Di, E. R. Guilmette, J. W. Schwartz, J. M. Fox, D. S. Johnson, J. Am. Chem. Soc. 2015, 137, 11461–11475;
- 19bM. Baalmann, M. J. Ziegler, P. Werther, J. Wilhelm, R. Wombacher, Bioconjugate Chem. 2019, 30, 1405–1414;
- 19cG. Beliu, A. J. Kurz, A. C. Kuhlemann, L. Behringer-Pliess, M. Meub, N. Wolf, J. Seibel, Z. D. Shi, M. Schnermann, J. B. Grimm, L. D. Lavis, S. Doose, M. Sauer, Commun. Biol. 2019, 2, 261;
- 19dA. Jemas, Y. Xie, J. E. Pigga, J. L. Caplan, C. W. am Ende, J. M. Fox, J. Am. Chem. Soc. 2022, 144, 1647–1662.
- 20
- 20aR. Rossin, P. R. Verkerk, S. M. van den Bosch, R. C. Vulders, I. Verel, J. Lub, M. S. Robillard, Angew. Chem. Int. Ed. 2010, 49, 3375–3378; Angew. Chem. 2010, 122, 3447–3450;
- 20bB. M. Zeglis, K. K. Sevak, T. Reiner, P. Mohindra, S. D. Carlin, P. Zanzonico, R. Weissleder, J. S. Lewis, J. Nucl. Med. 2013, 54, 1389–1396;
- 20cM. Wang, R. Vannam, W. D. Lambert, Y. Xie, H. Wang, B. Giglio, X. Ma, Z. Wu, J. Fox, Z. Li, Chem. Commun. 2019, 55, 2485–2488;
- 20dC. Wang, H. Zhang, T. Zhang, X. Zou, H. Wang, J. E. Rosenberger, R. Vannam, W. S. Trout, J. B. Grimm, L. D. Lavis, C. Thorpe, X. Jia, Z. Li, J. M. Fox, J. Am. Chem. Soc. 2021, 143, 10793–10803.
- 21
- 21aR. M. Versteegen, R. Rossin, W. ten Hoeve, H. M. Janssen, M. S. Robillard, Angew. Chem. Int. Ed. 2013, 52, 14112–14116; Angew. Chem. 2013, 125, 14362–14366;
- 21bA. van Onzen, R. M. Versteegen, F. J. M. Hoeben, I. A. W. Filot, R. Rossin, T. Zhu, J. Wu, P. J. Hudson, H. M. Janssen, W. Ten Hoeve, M. S. Robillard, J. Am. Chem. Soc. 2020, 142, 10955–10963.
- 22
- 22aK. Kang, J. Park, E. Kim, Proteome Sci. 2016, 15, 15;
- 22bL. Qian, S. Pan, J. S. Lee, J. Ge, L. Li, S. Q. Yao, Chem. Commun. 2019, 55, 1092–1095.
- 23
- 23aR. F. Gamache, K. A. Zettlitz, W. K. Tsai, J. Collins, A. M. Wu, J. M. Murphy, Chem. Sci. 2020, 11, 1832–1838;
- 23bL. Xu, M. Raabe, M. M. Zegota, J. C. F. Nogueira, V. Chudasama, S. L. Kuan, T. Weil, Org. Biomol. Chem. 2020, 18, 1140–1147.
- 24
- 24aM. Macias-Contreras, H. He, K. N. Little, J. P. Lee, R. P. Campbell, M. Royzen, L. Zhu, Bioconjugate Chem. 2020, 31, 1370–1381;
- 24bM. Rashidian, E. J. Keliher, A. M. Bilate, J. N. Duarte, G. R. Wojtkiewicz, J. T. Jacobsen, J. Cragnolini, L. K. Swee, G. D. Victora, R. Weissleder, H. L. Ploegh, Proc. Natl. Acad. Sci. USA 2015, 112, 6146–6151;
- 24cJ. E. Glasgow, M. L. Salit, J. R. Cochran, J. Am. Chem. Soc. 2016, 138, 7496–7499.
- 25
- 25aR. J. Blizzard, D. R. Backus, W. Brown, C. G. Bazewicz, Y. Li, R. A. Mehl, J. Am. Chem. Soc. 2015, 137, 10044–10047;
- 25bH. S. Jang, S. Jana, R. J. Blizzard, J. C. Meeuwsen, R. A. Mehl, J. Am. Chem. Soc. 2020, 142, 7245–7249;
- 25cK. Lang, L. Davis, S. Wallace, M. Mahesh, D. J. Cox, M. L. Blackman, J. M. Fox, J. W. Chin, J. Am. Chem. Soc. 2012, 134, 10317–10320.
- 26
- 26aD. A. Lorenz, A. L. Garner, Chem. Commun. 2016, 52, 8267–8270;
- 26bS. I. Lim, J. Cho, I. Kwon, Chem. Commun. 2015, 51, 13607–13610;
- 26cM. Baalmann, L. Neises, S. Bitsch, H. Schneider, L. Deweid, P. Werther, N. Ilkenhans, M. Wolfring, M. J. Ziegler, J. Wilhelm, H. Kolmar, R. Wombacher, Angew. Chem. Int. Ed. 2020, 59, 12885–12893; Angew. Chem. 2020, 132, 12985–12993;
- 26dA. Rutkowska, T. Plass, J. E. Hoffmann, D. A. Yushchenko, S. Feng, C. Schultz, ChemBioChem 2014, 15, 1765–1768;
- 26eE. M. Van Fossen, R. M. Bednar, S. Jana, R. Franklin, J. Beckman, P. A. Karplus, R. A. Mehl, Sci. Adv. 2022, 8, eabm6909.
- 27
- 27aJ. A. G. van Buggenum, J. P. Gerlach, S. E. J. Tanis, M. Hogeweg, P. Jansen, J. Middelwijk, R. van der Steen, M. Vermeulen, H. G. Stunnenberg, C. A. Albers, K. W. Mulder, Nat. Commun. 2018, 9, 2384;
- 27bS. S. Agasti, Y. Wang, F. Schueder, A. Sukumar, R. Jungmann, P. Yin, Chem. Sci. 2017, 8, 3080–3091;
- 27cJ. A. van Buggenum, J. P. Gerlach, S. Eising, L. Schoonen, R. A. van Eijl, S. E. Tanis, M. Hogeweg, N. C. Hubner, J. C. van Hest, K. M. Bonger, K. W. Mulder, Sci. Rep. 2016, 6, 22675.
- 28
- 28aM. Yang, W. J. Song, Nat. Commun. 2019, 10, 5545;
- 28bJ. H. Mills, W. Sheffler, M. E. Ener, P. J. Almhjell, G. Oberdorfer, J. H. Pereira, F. Parmeggiani, B. Sankaran, P. H. Zwart, D. Baker, Proc. Natl. Acad. Sci. USA 2016, 113, 15012–15017;
- 28cM. Bersellini, G. Roelfes, Org. Biomol. Chem. 2017, 15, 3069–3073;
- 28dM. Kang, K. Light, H. W. Ai, W. Shen, C. H. Kim, P. R. Chen, H. S. Lee, E. I. Solomon, P. G. Schultz, ChemBioChem 2014, 15, 822–825.
- 29
- 29aO. Stetsiuk, A. Abherve, N. Avarvari, Dalton Trans. 2020, 49, 5759–5777;
- 29bW. Kaim, Coord. Chem. Rev. 2002, 230, 127–139.
- 30K. Kawamoto, S. C. Grindy, J. Liu, N. Holten-Andersen, J. A. Johnson, ACS Macro Lett. 2015, 4, 458–461.
- 31S. Eising, A. H. J. Engwerda, X. Riedijk, F. M. Bickelhaupt, K. M. Bonger, Bioconjugate Chem. 2018, 29, 3054–3059.
- 32T. S. Tang, H. W. Liu, K. K. Lo, Chem. Commun. 2017, 53, 3299–3302.
- 33
- 33aA. Maggi, E. Ruivo, J. Fissers, C. Vangestel, S. Chatterjee, J. Joossens, F. Sobott, S. Staelens, S. Stroobants, P. Van Der Veken, L. Wyffels, K. Augustyns, Org. Biomol. Chem. 2016, 14, 7544–7551;
- 33bD. Svatunek, M. Wilkovitsch, L. Hartmann, K. N. Houk, H. Mikula, J. Am. Chem. Soc. 2022, 144, 8171–8177.
- 34N. E. Good, G. D. Winget, W. Winter, T. N. Connolly, S. Izawa, R. M. Singh, Biochemistry 1966, 5, 467–477.
- 35J. A. Bornhorst, J. J. Falke, Methods Enzymol. 2000, 326, 245–254.
- 36L. Tang, R. M. Bednar, N. D. Rozanov, M. L. Hemshorn, R. A. Mehl, C. Fang, Natural Sciences 2022, e20220028.
- 37M. Schnierle, S. Blickle, V. Filippou, M. R. Ringenberg, Chem. Commun. 2020, 56, 12033–12036.
- 38N. Pishesha, J. R. Ingram, H. L. Ploegh, Annu. Rev. Cell Dev. Biol. 2018, 34, 163–188.
- 39V. Agouridas, O. El Mahdi, V. Diemer, M. Cargoet, J. M. Monbaliu, O. Melnyk, Chem. Rev. 2019, 119, 7328–7443.