Two Cryptic Self-Resistance Mechanisms in Streptomyces tenebrarius Reveal Insights into the Biosynthesis of Apramycin
Graphical Abstract
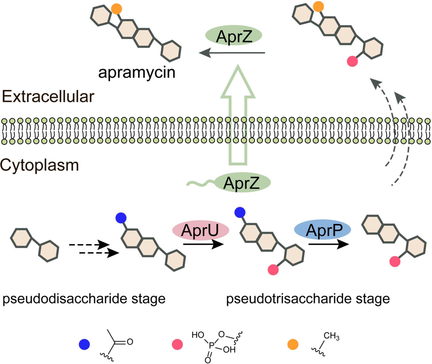
Two cryptic self-resistance mechanisms involving C-5 phosphorylation and N-7′ acetylation were discovered in Streptomyces tenebrarius to avoid auto-toxicity during apramycin biosynthesis. Characterization of the corresponding enzymes revealed the apramycin's assembly line at the pseudotrisaccharide stage.
Abstract
Apramycin is a clinically promising aminoglycoside antibiotic (AGA). To date, mechanisms underlying the biosynthesis and self-resistance of apramycin remain largely unknown. Here we report that apramycin biosynthesis proceeds through unexpected phosphorylation, deacetylation, and dephosphorylation steps, in which a novel aminoglycoside phosphotransferase (AprU), a putative creatinine amidohydrolase (AprP), and an alkaline phosphatase (AprZ) are involved. Biochemical characterization revealed that AprU specifically phosphorylates 5-OH of a pseudotrisaccharide intermediate, whose N-7′ acetyl group is subsequently hydrolyzed by AprP. AprZ is located extracellularly where it removes the phosphate group from a pseudotetrasaccharide intermediate, leading to the maturation of apramycin. Intriguingly, 7′-N-acetylated and 5-O-phosphorylated apramycin that were accumulated in ΔaprU and ΔaprZ respectively exhibited significantly reduced antibacterial activities, implying Streptomyces tenebrarius employs C-5 phosphorylation and N-7′ acetylation as two strategies to avoid auto-toxicity. Significantly, this study provides insight into the design of new generation AGAs to circumvent the emergence of drug-resistant pathogens.
Conflict of interest
The authors declare no conflict of interest.