Catalytic Enantioselective Desymmetrizing Fischer Indolization through Dynamic Kinetic Resolution
Biki Ghosh
Department of Organic Chemistry, Indian Institute of Science, Bangalore, 560 012 India
Search for more papers by this authorReena Balhara
Department of Organic Chemistry, Indian Institute of Science, Bangalore, 560 012 India
Search for more papers by this authorCorresponding Author
Prof. Dr. Garima Jindal
Department of Organic Chemistry, Indian Institute of Science, Bangalore, 560 012 India
Search for more papers by this authorCorresponding Author
Prof. Dr. Santanu Mukherjee
Department of Organic Chemistry, Indian Institute of Science, Bangalore, 560 012 India
Search for more papers by this authorBiki Ghosh
Department of Organic Chemistry, Indian Institute of Science, Bangalore, 560 012 India
Search for more papers by this authorReena Balhara
Department of Organic Chemistry, Indian Institute of Science, Bangalore, 560 012 India
Search for more papers by this authorCorresponding Author
Prof. Dr. Garima Jindal
Department of Organic Chemistry, Indian Institute of Science, Bangalore, 560 012 India
Search for more papers by this authorCorresponding Author
Prof. Dr. Santanu Mukherjee
Department of Organic Chemistry, Indian Institute of Science, Bangalore, 560 012 India
Search for more papers by this authorGraphical Abstract
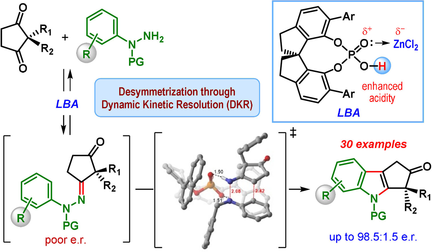
The first catalytic enantioselective desymmetrizing Fischer indolization of prochiral diketones, containing enantiotopic carbonyl groups, is developed and shown to proceed through dynamic kinetic resolution. Catalyzed by a combination of a spirocyclic chiral phosphoric acid and ZnCl2, this reaction delivers cyclopenta[b]indolones, containing an all-carbon quaternary stereocenter, with up to 98.5:1.5 e.r.
Abstract
The first catalytic enantioselective Fischer indolization of prochiral diketones containing enantiotopic carbonyl groups is developed and shown to proceed through dynamic kinetic resolution (DKR). Catalyzed by the combination of a spirocyclic chiral phosphoric acid and ZnCl2 (Lewis acid assisted Brønsted acid), this direct approach combines 2,2-disubstituted cyclopentane-1,3-diones with N-protected phenylhydrazines to furnish cyclopenta[b]indole derivatives containing an all-carbon quaternary stereocenter with good to excellent enantioselectivities.
Conflict of interest
The authors declare no conflict of interest.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202017268-sup-0001-misc_information.pdf11.4 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For selected reviews on indole synthesis, see:
- 1aA. K. Clarke, H. E. Ho, J. A. Rossi-Ashton, R. J. K. Taylor, W. P. Unsworth, Chem. Asian J. 2019, 14, 1900–1911;
- 1bD. I. Bugaenko, A. V. Karchava, M. A. Yurovskaya, Russ. Chem. Rev. 2019, 88, 99–159;
- 1cR. Mancuso, R. Dalpozzo, Catalysts 2018, 8, 458;
- 1dG. Bartoli, R. Dalpozzo, M. Nardi, Chem. Soc. Rev. 2014, 43, 4728–4750;
- 1eN. Yoshikai, Y. Wei, Asian J. Org. Chem. 2013, 2, 466–478;
- 1fM. Inman, C. J. Moody, Chem. Sci. 2013, 4, 29–41;
- 1gR. Vicente, Org. Biomol. Chem. 2011, 9, 6469–6480;
- 1hD. F. Taber, P. K. Tirunahari, Tetrahedron 2011, 67, 7195–7210;
- 1iG. R. Humphrey, J. T. Kuethe, Chem. Rev. 2006, 106, 2875–2911;
- 1jS. Cacchi, G. Fabrizi, Chem. Rev. 2005, 105, 2873–2920.
- 2For seminal reports, see:
- 2aE. Fischer, O. Hess, Ber. Dtsch. Chem. Ges. 1884, 17, 559–568;
10.1002/cber.188401701155 Google Scholar
- 2bE. Fischer, F. Jourdan, Ber. Dtsch. Chem. Ges. 1883, 16, 2241–2245; For reviews, see:
10.1002/cber.188301602141 Google Scholar
- 2cD. L. Hughes, Org. Prep. Proced. Int. 1993, 25, 607–632;
- 2dB. Robinson, The Fischer Indole Synthesis, Wiley-Interscience: New York, 1982;
- 2eB. Robinson, Chem. Rev. 1969, 69, 227–250;
- 2fB. Robinson, Chem. Rev. 1963, 63, 373–401.
- 3For selected recent examples, see:
- 3aA. Dierks, M. Schmidtmann, J. Christoffers, Chem. Eur. J. 2019, 25, 5451–5462;
- 3bA. W. Schammel, B. W. Boal, L. Zu, T. Mesganaw, N. K. Garg, Tetrahedron 2010, 66, 4687–4695;
- 3cB. W. Boal, A. W. Schammel, N. K. Garg, Org. Lett. 2009, 11, 3458–3461;
- 3dK. G. Liu, A. J. Robichaud, Tetrahedron Lett. 2007, 48, 461–463;
- 3eK. G. Liu, A. J. Robichaud, J. R. Lo, J. F. Mattes, Y. Cai, Org. Lett. 2006, 8, 5769–5771; For a review, see:
- 3fM. M. Heravi, S. Rohani, V. Zadsirjan, N. Zahedi, RSC Adv. 2017, 7, 52852–52887.
- 4For selected reviews on chiral phosphoric acids, see:
- 4aR. Maji, S. C. Mallojjala, S. E. Wheeler, Chem. Soc. Rev. 2018, 47, 1142–1158;
- 4bD. Parmar, E. Sugiono, S. Raja, M. Rueping, Chem. Rev. 2014, 114, 9047–9153;
- 4cM. Terada, Synthesis 2010, 1929–1982;
- 4dT. Akiyama, Chem. Rev. 2007, 107, 5744–5758.
- 5S. Müller, M. J. Webber, B. List, J. Am. Chem. Soc. 2011, 133, 18534–18537.
- 6
- 6aL. Kötzner, M. Leutzsch, S. Sievers, S. Patil, H. Waldmann, Y. Zheng, W. Thiel, B. List, Angew. Chem. Int. Ed. 2016, 55, 7693–7697; Angew. Chem. 2016, 128, 7824–7828;
- 6bL. Kötzner, M. J. Webber, A. Martínez, C. De Fusco, B. List, Angew. Chem. Int. Ed. 2014, 53, 5202–5205; Angew. Chem. 2014, 126, 5303–5306;
- 6cA. Martínez, M. J. Webber, S. Müller, B. List, Angew. Chem. Int. Ed. 2013, 52, 9486–9490; Angew. Chem. 2013, 125, 9664–9668.
- 7
- 7aT. Das, ChemistrySelect 2020, 5, 14484–14509;
- 7bX.-P. Zeng, Z.-Y. Cao, Y.-H. Wang, F. Zhou, J. Zhou, Chem. Rev. 2016, 116, 7330–7396;
- 7cA. Borissov, T. Q. Davies, S. R. Ellis, T. A. Fleming, M. S. W. Richardson, D. J. Dixon, Chem. Soc. Rev. 2016, 45, 5474–5540;
- 7dM. S. Manna, S. Mukherjee, Org. Biomol. Chem. 2015, 13, 18–24;
- 7eH. Fernández-Pérez, P. Etayo, J. R. Lao, J. L. Núñez-Rico, A. Vidal-Ferran, Chem. Commun. 2013, 49, 10666–10675.
- 8For selected examples, see:
- 8aG. R. Genov, J. L. Douthwaite, A. S. K. Lahdenperä, D. C. Gibson, R. J. Phipps, Science 2020, 367, 1246–1251;
- 8bH. Wu, Q. Wang, J. Zhu, Angew. Chem. Int. Ed. 2018, 57, 2721–2725; Angew. Chem. 2018, 130, 2751–2755;
- 8cH. Shi, A. N. Herron, Y. Shao, Q. Shao, J.-Q. Yu, Nature 2018, 558, 581–585;
- 8dA. J. Chinn, B. Kim, Y. Kwon, S. J. Miller, J. Am. Chem. Soc. 2017, 139, 18107–18114;
- 8eB. Su, T.-G. Zhou, P.-L. Xu, Z.-J. Shi, J. F. Hartwig, Angew. Chem. Int. Ed. 2017, 56, 7205–7208; Angew. Chem. 2017, 129, 7311–7314;
- 8fW. Luo, L. Lin, Y. Zhang, X. Liu, X. Feng, Org. Lett. 2017, 19, 3374–3377;
- 8gB. Kim, A. J. Chinn, D. R. Fandrick, C. H. Senanayake, R. A. Singer, S. J. Miller, J. Am. Chem. Soc. 2016, 138, 7939–7945;
- 8hZ. Huang, X. Huang, B. Li, C. Mou, S. Yang, B.-A. Song, Y. R. Chi, J. Am. Chem. Soc. 2016, 138, 7524–7527;
- 8iF. Zhou, C. Tan, J. Tang, Y.-Y. Zhang, W.-M. Gao, H.-H. Wu, Y.-H. Yu, J. Zhou, J. Am. Chem. Soc. 2013, 135, 10994–10997;
- 8jC. A. Lewis, J. L. Gustafson, A. Chiu, J. Balsells, D. Pollard, J. Murry, R. A. Reamer, K. B. Hansen, S. J. Miller, J. Am. Chem. Soc. 2008, 130, 16358–16365;
- 8kD. R. Cefalo, A. F. Kiely, M. Wuchrer, J. Y. Jamieson, R. R. Schrock, A. H. Hoveyda, J. Am. Chem. Soc. 2001, 123, 3139–3140; For a review, see:
- 8lA. J. Metrano, S. J. Miller, Acc. Chem. Res. 2019, 52, 199–215.
- 9
- 9aM. S. Manna, R. Sarkar, S. Mukherjee, Chem. Eur. J. 2016, 22, 14912–14919;
- 9bR. Sarkar, S. Mukherjee, Org. Lett. 2016, 18, 6160–6163;
- 9cM. S. Manna, S. Mukherjee, J. Am. Chem. Soc. 2015, 137, 130–133.
- 10
- 10aK. W. Quasdorf, L. E. Overman, Nature 2014, 516, 181–191;
- 10bB. M. Trost, C. Jiang, Synthesis 2006, 369–396;
- 10cC. J. Douglas, L. E. Overman, Proc. Natl. Acad. Sci. USA 2004, 101, 5363–5367.
- 11
- 11aP. Yi, J. F. Rehmel, K. Cassidy, C. Hadden, K. Campanale, N. Patel, J. Johnson, Drug Metab. Dispos. 2012, 40, 2354–2364;
- 11bH. Chen, J. Bai, Z.-F. Fang, S.-S. Yu, S.-G. Ma, S. Xu, Y. Li, J. Qu, J.-H. Ren, L. Li, Y.-K. Si, X.-G. Chen, J. Nat. Prod. 2011, 74, 2438–2445;
- 11cJ. Nakazawa, J. Yajima, T. Usui, M. Ueki, A. Takatsuki, M. Imoto, Y. Y. Toyoshima, H. Osada, Chem. Biol. 2003, 10, 131–137;
- 11dA. Ploutno, S. Carmeli, J. Nat. Prod. 2001, 64, 544–545;
- 11eY.-C. Kong, K.-F. Cheng, R. C. Cambie, P. G. Waterman, J. Chem. Soc. Chem. Commun. 1985, 47–48;
- 11fJ. P. Springer, J. Clardy, Tetrahedron Lett. 1980, 21, 231–234.
- 12For selected examples, see:
- 12aJ.-L. Wu, J.-Y. Wang, P. Wu, J.-R. Wang, G.-J. Mei, F. Shi, Org. Chem. Front. 2018, 5, 1436–1445;
- 12bM.-M. Xu, H.-Q. Wang, Y. Wan, S.-L. Wang, F. Shi, J. Org. Chem. 2017, 82, 10226–10233;
- 12cM. A. Abozeid, S. Sairenji, S. Takizawa, M. Fujita, H. Sasai, Chem. Commun. 2017, 53, 6887–6890;
- 12dC.-Y. Wu, Y.-N. Yu, M.-H. Xu, Org. Lett. 2017, 19, 384–387;
- 12eW. Zi, H. Wu, F. D. Toste, J. Am. Chem. Soc. 2015, 137, 3225–3228;
- 12fB. Xu, Z. L. Guo, W. Y. Jin, Z. P. Wang, Y. G. Peng, Q. X. Guo, Angew. Chem. Int. Ed. 2012, 51, 1059–1062; Angew. Chem. 2012, 124, 1083–1086;
- 12gB. Han, Y.-C. Xiao, Y. Yao, Y.-C. Chen, Angew. Chem. Int. Ed. 2010, 49, 10189–10191; Angew. Chem. 2010, 122, 10387–10389;
- 12hY. Lian, H. M. L. Davies, J. Am. Chem. Soc. 2010, 132, 440–441;
- 12iJ. A. Schiffner, T. H. Wöste, M. Oestreich, Eur. J. Org. Chem. 2010, 174–182;
- 12jJ. Barluenga, E. Tudela, A. Ballesteros, M. Tomás, J. Am. Chem. Soc. 2009, 131, 2096–2097;
- 12kJ. A. Schiffner, A. B. Machotta, M. Oestreich, Synlett 2008, 2271–2274;
- 12lK. R. Campos, M. Journet, S. Lee, E. J. J. Grabowski, R. D. Tillyer, J. Org. Chem. 2005, 70, 268–274; For a review, see:
- 12mT. Vivekanand, B. Satpathi, S. K. Bankar, S. S. V. Ramasastry, RSC Adv. 2018, 8, 18576–18588.
- 13
- 13aC. Uyeda, E. N. Jacobsen, J. Am. Chem. Soc. 2011, 133, 5062–5075;
- 13bC. Uyeda, A. R. Rötheli, E. N. Jacobsen, Angew. Chem. Int. Ed. 2010, 49, 9753–9756; Angew. Chem. 2010, 122, 9947–9950;
- 13cM. Rueping, A. P. Antonchick, Angew. Chem. Int. Ed. 2008, 47, 10090–10093; Angew. Chem. 2008, 120, 10244–10247;
- 13dC. Uyeda, E. N. Jacobsen, J. Am. Chem. Soc. 2008, 130, 9228–9229.
- 14
- 14aK. Faber, Chem. Eur. J. 2001, 7, 5004–5010;
10.1002/1521-3765(20011203)7:23<5004::AID-CHEM5004>3.0.CO;2-X CAS PubMed Web of Science® Google Scholar
- 14bJ. M. Keith, J. F. Larrow, E. N. Jacobsen, Adv. Synth. Catal. 2001, 343, 5–26.
- 15For seminal reports on spirocyclic chiral phosphoric acid, see:
- 15aI. Čorić, S. Müller, B. List, J. Am. Chem. Soc. 2010, 132, 17370–17373;
- 15bF. Xu, D. Huang, C. Han, W. Shen, X. Lin, Y. Wang, J. Org. Chem. 2010, 75, 8677–8680; For a review, see:
- 15cA. Rahman, X. Lin, Org. Biomol. Chem. 2018, 16, 4753–4777.
- 16For the use of Amberlite IR120 in Fischer indole synthesis, see: S. Chandrasekhar, S. Mukherjee, Synth. Commun. 2015, 45, 1018–1022.
- 17For details, see the Supporting Information.
- 18H. Yamamoto, K. Futatsugi, Angew. Chem. Int. Ed. 2005, 44, 1924–1942; Angew. Chem. 2005, 117, 1958–1977.
- 19
- 19aM. Hatano, T. Sakamoto, T. Mochizuki, K. Ishihara, Asian J. Org. Chem. 2019, 8, 1061–1066;
- 19bT. Sakamoto, T. Mochizuki, Y. Goto, M. Hatano, K. Ishihara, Chem. Asian J. 2018, 13, 2373–2377;
- 19cM. Hatano, Y. Goto, A. Izumiseki, M. Akakura, K. Ishihara, J. Am. Chem. Soc. 2015, 137, 13472–13475; Also see:
- 19dQ.-X. Zhang, Y. Li, J. Wang, C. Yang, C.-J. Liu, X. Li, J.-P. Cheng, Angew. Chem. Int. Ed. 2020, 59, 4550–4556; Angew. Chem. 2020, 132, 4580–4586.
- 20For the use of ZnCl2 in Fischer indolization, see:
- 20aB.-Y. Lim, B.-E. Jung, C.-G. Cho, Org. Lett. 2014, 16, 4492–4495;
- 20bT. M. Lipińska, S. J. Czarnocki, Org. Lett. 2006, 8, 367–370.
- 21J. M. Lopchuk, I. L. Green, J. C. Badenock, G. W. Gribble, Org. Lett. 2013, 15, 4485–4487.
- 22
- 22aGaussian 16, Revision C.01, M. J. Frisch, et al., Gaussian, Inc., Wallingford, CT, 2019;
- 22bY. Zhao, D. G. Truhlar, Theor. Chem. Acc. 2008, 120, 215–241;
- 22cSee the Supporting Information for full computational details.
- 23
- 23aT. J. Seguin, T. Lu, S. E. Wheeler, Org. Lett. 2015, 17, 3066–3069; Also see:
- 23bN. Çelebi-Ölçüm, B. W. Boal, A. D. Huters, N. K. Garg, K. N. Houk, J. Am. Chem. Soc. 2011, 133, 5752–5755.
- 24For complete details on the conformational study, see Table S8 of the Supporting Information.
- 25F. M. Bickelhaupt, K. N. Houk, Angew. Chem. Int. Ed. 2017, 56, 10070–10086; Angew. Chem. 2017, 129, 10204–10221.