Hydrogen-Bonding Assisted Catalytic Kinetic Resolution of Acyclic β-Hydroxy Amides
Arka Porey
School of Chemical Sciences, Indian Association for the Cultivation of Science, 2A & 2B Raja S. C. Mullick Road, Jadavpur, Kolkata, 700032 India
Search for more papers by this authorBhaskar Deb Mondal
School of Chemical Sciences, Indian Association for the Cultivation of Science, 2A & 2B Raja S. C. Mullick Road, Jadavpur, Kolkata, 700032 India
Search for more papers by this authorCorresponding Author
Dr. Joyram Guin
School of Chemical Sciences, Indian Association for the Cultivation of Science, 2A & 2B Raja S. C. Mullick Road, Jadavpur, Kolkata, 700032 India
Search for more papers by this authorArka Porey
School of Chemical Sciences, Indian Association for the Cultivation of Science, 2A & 2B Raja S. C. Mullick Road, Jadavpur, Kolkata, 700032 India
Search for more papers by this authorBhaskar Deb Mondal
School of Chemical Sciences, Indian Association for the Cultivation of Science, 2A & 2B Raja S. C. Mullick Road, Jadavpur, Kolkata, 700032 India
Search for more papers by this authorCorresponding Author
Dr. Joyram Guin
School of Chemical Sciences, Indian Association for the Cultivation of Science, 2A & 2B Raja S. C. Mullick Road, Jadavpur, Kolkata, 700032 India
Search for more papers by this authorGraphical Abstract
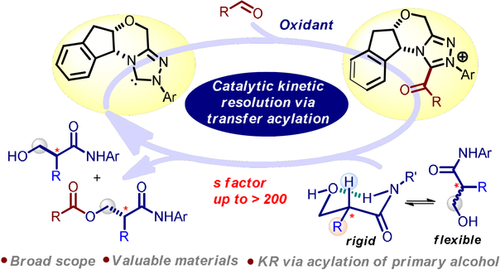
A method for catalytic kinetic resolution (KR) of acyclic α-substituted β-hydroxy amides was developed via enantioselective acylation of primary alcohol with an N-heterocyclic carbene. Enhanced selectivity was realized for the catalytic KR process using cyclic tertiary amine as a base additive. Diastereomeric transition state models are proposed to rationalize the origin of enantioselectivity.
Abstract
Enantioenriched acyclic α-substituted β-hydroxy amides are valuable compounds in chemical, material and medicinal sciences, but their enantioselective synthesis remains challenging. A catalytic kinetic resolution (KR) of such amides with selectivity factor(s) up to >200 is developed via enantioselective acylation of primary alcohol with N-heterocyclic carbene. An enhanced selectivity for the catalytic KR process is realized using cyclic tertiary amine as base additive. Diastereomeric transition state models for the process are proposed to rationalize the origin of enantioselectivity.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202015004-sup-0001-misc_information.pdf12.3 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1P. Buehlmayer, A. Caselli, W. Fuhrer, R. Goeschke, V. Rasetti, H. Rueger, J. L. Stanton, L. Criscione, J. M. Wood, J. Med. Chem. 1988, 31, 1839.
- 2
- 2aD. Fiorito, Y. Liu, C. Besnard, C. Mazet, J. Am. Chem. Soc. 2020, 142, 623;
- 2bR. Marín-Valls, K. Hernández, M. Bolte, J. Joglar, J. Bujons, P. Clapés, ACS Catal. 2019, 9, 7568;
- 2cH. Tokuyama, K. Yamada, H. Fujiwara, J. Sakata, K. Okano, M. Sappan, M. Isaka, J. Org. Chem. 2017, 82, 353;
- 2dÁ. Enríquez-García, E. P. Kündig, Chem. Soc. Rev. 2012, 41, 7803;
- 2eO. Vergnolle, F. Hahn, A. Baerga-Ortiz, P. F. Leadlay, J. N. Andexer, ChemBioChem 2011, 12, 1011;
- 2fF. Sarabia, S. Chammaa, C. García-Ruiz, J. Org. Chem. 2011, 76, 2132;
- 2gY. Hamashima, S. Suzuki, T. Tamura, H. Somei, M. Sodeoka, Chem. Asian J. 2011, 6, 658;
- 2hR. K. Boeckman, J. R. Miller, Org. Lett. 2009, 11, 4544;
- 2iH. S. Lee, J.-D. Park, Bull. Korean Chem. Soc. 2003, 24, 467;
- 2jD. A. Evans, E. B. Sjogren, J. Bartroli, R. L. Dow, Tetrahedron Lett. 1986, 27, 4957.
- 3
- 3aL. Zhang, Z. Wang, Z. Han, K. Ding, Angew. Chem. Int. Ed. 2020, 59, 15565; Angew. Chem. 2020, 132, 15695;
- 3bD. Méndez-Sánchez, Á. Mourelle-Insua, V. Gotor-Fernández, I. Lavandera, Adv. Synth. Catal. 2019, 361, 2706;
- 3cT.-T. Gao, W.-W. Zhang, X. Sun, H.-X. Lu, B.-J. Li, J. Am. Chem. Soc. 2019, 141, 4670;
- 3dS. De, M. K. Das, A. Roy, A. Bisai, J. Org. Chem. 2016, 81, 12258;
- 3eS. H. Shin, E. H. Baek, G.-S. Hwang, D. H. Ryu, Org. Lett. 2015, 17, 4746;
- 3fG. Kumaraswamy, A. Narayana Murthy, V. Narayanarao, S. P. B. Vemulapalli, J. Bharatam, Org. Biomol. Chem. 2013, 11, 6751;
- 3gX.-L. Liu, Y.-H. Liao, Z.-J. Wu, L.-F. Cun, X.-M. Zhang, W.-C. Yuan, J. Org. Chem. 2010, 75, 4872;
- 3hJ. Limanto, S. W. Krska, B. T. Dorner, E. Vazquez, N. Yoshikawa, L. Tan, Org. Lett. 2010, 12, 512;
- 3iM. Quirós, F. Rebolledo, V. Gotor, Tetrahedron: Asymmetry 1999, 10, 473.
- 4
- 4aN. Kumagai, M. Shibasaki, Synthesis 2019, 51, 185;
- 4bS. Rossi, M. Benaglia, F. Cozzi, A. Genoni, T. Benincori, Adv. Synth. Catal. 2011, 353, 848;
- 4cH. M. L. Davies, S. J. Hedley, B. R. Bohall, J. Org. Chem. 2005, 70, 10737;
- 4dF. Molinari, R. Gandolfi, R. Villa, E. Urban, A. Kiener, Tetrahedron: Asymmetry 2003, 14, 2041;
- 4eD. A. Evans, J. Bartroli, T. L. Shih, J. Am. Chem. Soc. 1981, 103, 2127.
- 5Selected reviews on catalytic kinetic resolution:
- 5aH. Yang, W.-H. Zheng, Tetrahedron Lett. 2018, 59, 583;
- 5bH. Pellissier, Tetrahedron 2018, 74, 3459;
- 5cR. Gurubrahamam, Y.-S. Cheng, W.-Y. Huang, K. Chen, ChemCatChem 2016, 8, 86;
- 5dG. Ma, M. P. Sibi, Chem. Eur. J. 2015, 21, 11644;
- 5eC. E. Müller, P. R. Schreiner, Angew. Chem. Int. Ed. 2011, 50, 6012; Angew. Chem. 2011, 123, 6136;
- 5fE. Vedejs, M. Jure, Angew. Chem. Int. Ed. 2005, 44, 3974; Angew. Chem. 2005, 117, 4040;
- 5gD. E. J. E. Robinson, S. D. Bull, Tetrahedron: Asymmetry 2003, 14, 1407;
- 5hJ. M. Keith, J. F. Larrow, E. N. Jacobsen, Adv. Synth. Catal. 2001, 343, 5;
- 5iK. Faber, Chem. Eur. J. 2001, 7, 5004.
10.1002/1521-3765(20011203)7:23<5004::AID-CHEM5004>3.0.CO;2-X CAS PubMed Web of Science® Google Scholar
- 6
- 6aM. D. Greenhalgh, J. E. Taylor, A. D. Smith, Tetrahedron 2018, 74, 5554;
- 6bH. B. Kagan, J. C. Fiaud, Topics in Stereochemistry, Wiley, Hoboken, 1988, pp. 249.
10.1002/9780470147276.ch4 Google Scholar
- 7Recent reviews on transfer acylation chemistry involving acyl azolium intermediate:
- 7aS. Mondal, S. R. Yetra, S. Mukherjee, A. T. Biju, Acc. Chem. Res. 2019, 52, 425;
- 7bK. J. R. Murauski, A. A. Jaworski, K. A. Scheidt, Chem. Soc. Rev. 2018, 47, 1773;
- 7cC. Zhang, J. F. Hooper, D. W. Lupton, ACS Catal. 2017, 7, 2583;
- 7dD. M. Flanigan, F. Romanov-Michailidis, N. A. White, T. Rovis, Chem. Rev. 2015, 115, 9307;
- 7eJ. Mahatthananchai, J. W. Bode, Acc. Chem. Res. 2014, 47, 696;
- 7fM. N. Hopkinson, C. Richter, M. Schedler, F. Glorius, Nature 2014, 510, 485;
- 7gS. De Sarkar, A. Biswas, R. C. Samanta, A. Studer, Chem. Eur. J. 2013, 19, 4664;
- 7hJ. Douglas, G. Churchill, A. D. Smith, Synthesis 2012, 44, 2295.
- 8Recent reviews on NHC-catalyzed kinetic resolution:
- 8aC. De Risi, O. Bortolini, G. Di Carmine, D. Ragno, A. Massi, Synthesis 2019, 51, 1871;
- 8bZ. Wang, D. Pan, T. Li, Z. Jin, Chem. Asian J. 2018, 13, 2149.
- 9Selected examples of dynamic kinetic resolution via NHC-catalyzed transfer acylation:
- 9aB. Liu, R. Song, J. Xu, P. K. Majhi, X. Yang, S. Yang, Z. Jin, Y. R. Chi, Org. Lett. 2020, 22, 3335;
- 9bC. Zhao, F. Li, J. Wang, Angew. Chem. Int. Ed. 2016, 55, 1820; Angew. Chem. 2016, 128, 1852;
- 9cX. Chen, J. Z. M. Fong, J. Xu, C. Mou, Y. Lu, S. Yang, B.-A. Song, Y. R. Chi, J. Am. Chem. Soc. 2016, 138, 7212;
- 9dZ. Wu, F. Li, J. Wang, Angew. Chem. Int. Ed. 2015, 54, 1629; Angew. Chem. 2015, 127, 1649.
- 10S. De Sarkar, A. Biswas, C. H. Song, A. Studer, Synthesis 2011, 1974.
- 11S. Iwahana, H. Iida, E. Yashima, Chem. Eur. J. 2011, 17, 8009.
- 12J. Bie, M. Lang, J. Wang, Org. Lett. 2018, 20, 5866.
- 13S. Lu, S. B. Poh, Y. Zhao, Angew. Chem. Int. Ed. 2014, 53, 11041; Angew. Chem. 2014, 126, 11221.
- 14S. Lu, S. B. Poh, W.-Y. Siau, Y. Zhao, Angew. Chem. Int. Ed. 2013, 52, 1731; Angew. Chem. 2013, 125, 1775.
- 15S. Kuwano, S. Harada, B. Kang, R. Oriez, Y. Yamaoka, K. Takasu, K.-i. Yamada, J. Am. Chem. Soc. 2013, 135, 11485.
- 16B. Liu, J. Yan, R. Huang, W. Wang, Z. Jin, G. Zanoni, P. Zheng, S. Yang, Y. R. Chi, Org. Lett. 2018, 20, 3447.
- 17NHC-catalyzed kinetic resolution via N-acylation:
- 17aA. Brandolese, D. Ragno, C. Leonardi, G. Di Carmine, O. Bortolini, C. De Risi, A. Massi, Eur. J. Org. Chem. 2020, 2439;
- 17bS. Dong, M. Frings, H. Cheng, J. Wen, D. Zhang, G. Raabe, C. Bolm, J. Am. Chem. Soc. 2016, 138, 2166;
- 17cM. Binanzer, S.-Y. Hsieh, J. W. Bode, J. Am. Chem. Soc. 2011, 133, 19698.
- 18An interesting example of the catalytic enantioselective acyl transfer with primary alcohols: C. Roux, M. Candy, J.-M. Pons, O. Chuzel, C. Bressy, Angew. Chem. Int. Ed. 2014, 53, 766; Angew. Chem. 2014, 126, 785.
- 19
- 19aA. Cuetos, A. Rioz-Martínez, F. R. Bisogno, B. Grischek, I. Lavandera, G. de Gonzalo, W. Kroutil, V. Gotor, Adv. Synth. Catal. 2012, 354, 1743;
- 19bM. Kurina-Sanz, F. R. Bisogno, I. Lavandera, A. A. Orden, V. Gotor, Adv. Synth. Catal. 2009, 351, 1842;
- 19cL. Joris, P. v. R. Schleyer, J. Am. Chem. Soc. 1968, 90, 4599.
- 20
- 20aS. Santra, U. Maji, J. Guin, Org. Lett. 2020, 22, 468;
- 20bA. Porey, S. Santra, J. Guin, J. Org. Chem. 2019, 84, 5313;
- 20cS. Santra, A. Porey, B. Jana, J. Guin, Chem. Sci. 2018, 9, 6446.
- 21The selectivity factor(s) is calculated according to the following equation: s=ln[(1−c)(1−eeSM)]/ln[(1−c)(1+eeSM)], where the reaction conversion (c)=eeSM/(eeSM+eeP); eeP=ee of product and eeSM=ee of the recovered starting material. See Ref. [6] for more details.
- 22The hydrolysis of ester 6 f afforded the ent-5 fr with good enanti-oselectivity. Absolute configuration of both 5 fr and ent-5 fr was con-firmed by single crystal X-ray analysis of the corresponding mesyl derivatives 9 and ent-9 (see, the Supporting Information). This demonstartes that both enantiomers of the racemic substarte could be accessed with this catalytic KR process in good level of enantiomeric excess.
- 23B. Alcaide, P. Almendros, C. Aragoncillo, Chem. Rev. 2007, 107, 4437.
- 24A. Lorente, J. Lamariano-Merketegi, F. Albericio, M. Álvarez, Chem. Rev. 2013, 113, 4567.
- 25H. Barbero, C. Díez-Poza, A. Barbero, Mar. Drugs 2017, 15, 361.
- 26O. E. Hutt, B. S. Reddy, S. K. Nair, E. A. Reiff, J. T. Henri, J. F. Greiner, T.-L. Chiu, D. G. VanderVelde, E. A. Amin, R. H. Himes, G. I. Georg, Bioorg. Med. Chem. Lett. 2008, 18, 4904.
- 27
- 27aM. D. Greenhalgh, S. M. Smith, D. M. Walden, J. E. Taylor, Z. Brice, E. R. T. Robinson, C. Fallan, D. B. Cordes, A. M. Z. Slawin, H. C. Richardson, M. A. Grove, P. H.-Y. Cheong, A. D. Smith, Angew. Chem. Int. Ed. 2018, 57, 3200; Angew. Chem. 2018, 130, 3254;
- 27bE. Larionov, M. Mahesh, A. C. Spivey, Y. Wei, H. Zipse, J. Am. Chem. Soc. 2012, 134, 9390;
- 27cS. Xu, I. Held, B. Kempf, H. Mayr, W. Steglich, H. Zipse, Chem. Eur. J. 2005, 11, 4751.